Human hydatid disease, or echinococcosis, is a helminthic infection that leads to the formation of fluid-filled cysts in the liver, lungs and other organs. Echinococcus granulosus, which causes cystic echinococcosis, or unilocular cyst disease of viscera,1 is the only member of the genus Echinococcus to be found in Australia. It was introduced into Australia during the early period of European settlement and had been described in domestic animals before 1840.2
E. granulosus is a cyclozoonosis, requiring at least two species of vertebrates as definitive and intermediate hosts (Box 1). Dogs and other canids such as dingos and foxes are definitive hosts and infection occurs following ingestion of metacestodes (cysts) in mammalian organs, leading to the shedding of infective eggs containing larval oncospheres in the faeces. The intermediate host is infected following ingestion of infective eggs. The intermediate host range is broad and regionally specific.3 It includes domestic and feral ungulates such as sheep, goats, pigs, camels and buffaloes, and marsupials such as kangaroos and wallabies.4 This broad host range has resulted in the establishment of domestic and sylvatic cycles, which in some regions may intersect.
Humans are infected as intermediate hosts. Following egg ingestion, the oncosphere is absorbed through the intestinal wall and deposited via the circulatory system to various organs, with subsequent cyst formation. The most common sites of human disease are the liver (> 65%) and lungs (20%).3,5 Other less commonly affected areas include other intra-abdominal sites such as kidney, spleen, peritoneal cavity and, more rarely, spine, brain, heart and bone.3 Infection in some sites, particularly the liver, may remain asymptomatic for many years and is usually diagnosed well into adulthood, often incidentally. Conversely, disease in the central nervous system becomes symptomatic and presents much sooner after initial infection.
During the past century, Tasmania experienced one of the highest rates of human hydatid disease in the world,6,7 perpetuated by a hydatid life cycle involving dogs and sheep. Between 1957 and 1967, 28 fatal human cases were recorded.7 Estimates of human disease based on surgical cases in the 1950s and early 1960s ranged from 92.5 to 151 cases per 100 000 population per decade.8,9 At this time, the Australian annual rate was 1.6 per 100 000 human population nationally and 7.8 per 100 000 in rural areas.10 Surveys of slaughtered sheep in different Tasmanian regions in 1963 revealed a hydatid prevalence of 35%–73%,8 and surveys of rural dogs reported a prevalence of 12.7%.6
Increasing concern regarding the human health impact of hydatid disease led to the formation of the Tasmanian Hydatids Eradication Council in 1962.6 A systematic campaign commenced in 1965. This encompassed regular testing of dog faeces, anthelmintic treatment of dogs, examination of abattoir-slaughtered sheep, community education regarding safe slaughtering practices and farm hygiene, and the prohibition of feeding offal to dogs.6
The eradication campaign resulted in a rapid and significant reduction in the prevalence of hydatid infection among dogs and sheep,11 and the surgical incidence of hydatid disease among humans.12
From 30 July to 30 October 2012, systematic data collection was undertaken to identify cases of hydatid disease in patients who presented to medical practitioners in Tasmania from January 1996 to July 2012. Approval for the study was obtained from the Human Research Ethics Committee (Tasmania). Patients undergoing serological testing for hydatids were identified from the Royal Hobart Hospital Department of Pathology laboratory information system. Hospital admissions were identified from discharge coding data from all major Tasmanian public hospitals (Royal Hobart, Launceston General, Mersey Community and North West Regional) and the Royal Hobart Hospital Microbiology and Infectious Diseases Unit consultation database. Permission was obtained from the Director of Public Health to access the Department of Health and Human Services notifications for hydatid disease for the years 1996–2012.
Fifty-one patients with possible cases of hydatid infection were identified, of whom 41 fulfilled the case definition. Median age for patients was 71 years (range, 44–99 years). There were 21 women and 20 men. Patient demographics and clinical features are summarised in Box 2. Two patients were born after 1965: one in 1967 and one in 1968.
From January 1996 to July 2012, 25 patients were diagnosed with hydatid disease, with an average rate of 1.3 cases per year (range, 0–3 cases per year) (Box 3). Of the 25 patients, 10 (40%) had been notified to the Department of Health and Human Services.
Among these, there were no cases in children, no cases of extra-abdominal disease and only four cases of extrahepatic disease (Box 2).
Our study has found no evidence of hydatid transmission in Tasmania between 1996, the year that the state was provisionally declared hydatid-free, and 2012. A preponderance of hepatic disease and paucity of childhood and extrahepatic disease, as found in this study, is observed in regions where local transmission has ceased.6,13
It has previously been estimated that human hydatid transmission had ceased in Tasmania by 1974, possibly by as early as 1970.6,14 This conclusion was based on data that demonstrated the absence of hydatid disease in children born shortly after the program was introduced. The trend of increased median age at diagnosis since 1965 among study subjects (Box 2) and the fact that no subject was born after 1968 support the suggestion that human hydatid acquisition ceased well before 1996. All but one of the 29 patients assessed for time of hydatid acquisition described risk factors before the mid 1970s. The remaining patient moved to a sheep-farming region in the late 1970s, at which time the prevalence across the state in rural dogs was estimated at 0.2%.11
E. granulosus infection continues to occur widely in mainland Australia, predominantly in cooler regions with high rainfall, and it is estimated that 80–100 patients are diagnosed with hydatid infection per year in Australia.15 A survey of New South Wales and the Australian Capital Territory from 1987 to 1992 found the highest prevalence in the shire population of north-east and south-east NSW, with a mean annual prevalence of up to 23.5 cases per 100 000 population,15 a rate comparable to that of rural Tasmania before eradication. The sylvatic life cycle established in many mainland regions will be a significant obstacle to eradication from mainland Australia.2,15-18
Estimating the true prevalence of human hydatid disease in Australia is hampered by the poor quality of notification data, which has been repeatedly demonstrated to underestimate the true incidence of the disease.15 Our study found a notification rate of just 40%. Reasons for such poor notification rates are likely to be multifactorial. However, a lack of awareness of the requirement to notify cases and the perception that human hydatid disease is no longer a disease of public health importance in Tasmania are likely to be contributing factors.
The threat of recurrence in Tasmania remains. A new focus of infection was identified in 1988 on King Island, north of Tasmania, where hydatids had not been detected since 1971.19 The source of the outbreak was never identified, and typing of the isolates found them to be genetically distinct from Tasmanian and mainland Australian strains. In 1997, a single sheep and cow were found to have hydatid cysts.9 The source was traced back to a dog that had been introduced from mainland Australia. Since then it has been mandatory to dose any dog being brought to Tasmania with praziquantel before arrival. It remains illegal to feed offal to dogs.
1 Life cycle of Echinococcus granulosus
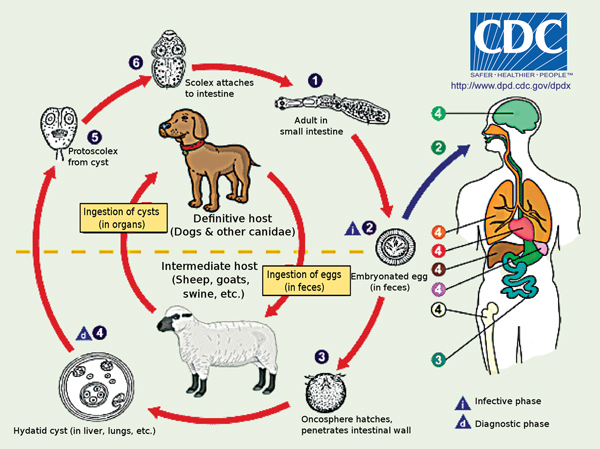
Source: DPDx Laboratory Identification of Parasites of Public Health Concern. Echinococcosis [internet image library]. Atlanta: Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention. http://dpd.cdc.gov/dpdx/HTML/ImageLibrary/Echinococcosis_il.htm (accessed Feb 2013). The adult worm resides in the small intestine of the definitive host (1). It releases eggs into the environment (2), which are then ingested by an intermediate host. In the small intestine of the intermediate host, the oncosphere hatches, penetrates the intestinal wall (3) to enter the bloodstream, whereby it migrates and is deposited in other organs (4) and forms a cyst. The cyst produces protoscoleces (5). The cycle is complete when the intermediate host is eaten by a definitive host and ingests the protoscoleces, which go on to develop into the adult worm.
2 Patient demographics and echinococcosis infection site details, by years of diagnosis
Derwent Valley (1); central (1); south (1); overseas (1); rural: not specified (1); unknown (4) |
|||||||||||||||
3 Number of new echinococcosis diagnoses per year in Tasmania, 1967–2012
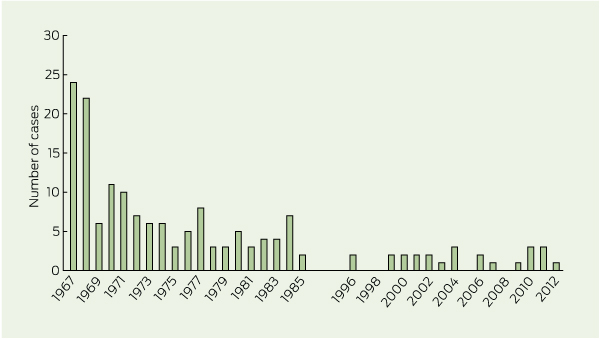
Data for diagnoses from 1967 (2 years after introduction of the eradication program) to 1985 sourced from McConnell11 and Tasmanian Hydatid Disease Newsletter 1971; 3(31) (Tasmanian Hydatids Eradication Council). Data for diagnoses from 1996 onward collected in this study.
Received 29 November 2012, accepted 25 March 2013
- Jennifer A O’Hern1
- Louise Cooley2
- Royal Hobart Hospital, Hobart, TAS.
We thank David Coleman, Population Health, Department of Human Health and Services; the Business Intelligence Unit, Royal Hobart Hospital and North West Regional Hospital; Patient Information Management Services, Royal Hobart Hospital and Launceston General Hospital; David Jones, Department of Microbiology, Royal Hobart Hospital; Clinical Classification and Information, Royal Hobart Hospital; and the Medical Records Department, North West Regional Hospital.
No relevant disclosures.
- 1. King CH, Fairley JK. Cestodes (tapeworms). In: Mandell GM, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 7th ed. Philadelphia: Elsevier, 2010.
- 2. Gemmell MA. Australasian contributions to an understanding of the epidemiology and control of hydatid disease caused by Echinococcus granulosus--past, present and future. Int J Parasitol 1990; 20: 431-456.
- 3. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004; 17: 107-135.
- 4. Jenkins DJ, Macpherson CN. Transmission ecology of Echinococcus in wild-life in Australia and Africa. Parasitology 2003; 127 Suppl: S63-S72.
- 5. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362: 1295-1304.
- 6. Beard TC, Bramble AJ, Middleton MJ. Eradication in our lifetime. A log book of the Tasmanian Hydatid Control Programs, 1962-1996. Hobart: Department of Primary Industry, Water and Environment, 2001.
- 7. Beard TC. Hydatid disease. Aust J Physiother 1964; 10: 24-26. (accessed Mar 2013).
- 8. Meldrum GK, McConnell JD. The control of hydatid disease in Tasmania. Aust Vet J 1968; 44: 212-217.
- 9. Craig PS, Larrieu E. Control of cystic echinococcosis hydatidosis: 1863-2002. Adv Parasitol 2006; 61: 443-508.
- 10. Dew HR. Hydatid disease in Australia. Arch Internac Hidatid 1953; 13: 319-332.
- 11. McConnell JD. Hydatid disease in Tasmania: control in animals. In: King H, editor. Epidemiology in Tasmania. Canberra: Brolga Press, 1987: 61-75.
- 12. Beard TC. Evidence that a hydatid cyst is seldom “as old as the patient”. Lancet 1978; 2: 30-32.
- 13. Craig PS, McManus DP, Lightowlers MW, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis 2007; 7: 385-394.
- 14. Beard TC. Human behaviour and the ethics of coercion. Med J Aust 1988; 148: 82-85.
- 15. Jenkins D. Cystic echinococcosis in Australia: the current situation. Southeast Asian J Trop Med Public Health 2004; 35: 183-188.
- 16. Jenkins DJ, Power K. Human hydatidosis in New South Wales and the Australian Capital Territory, 1987-1992. Med J Aust 1996; 164: 18-21.
- 17. McCullagh PJ. Hydatid disease: medical problems, veterinary solutions, political obstacles. Med J Aust 1996; 164: 7-8.
- 18. Jenkins DJ, Morris B. Echinococcus granulosus in wildlife in and around the Kosciusko National Park, south-eastern Australia. Aust Vet J 2003; 81: 81-85.
- 19. Constantine CC, Lymbery AJ, Thompson RC, Obendorf DL. The origin of a new focus of infection with Echinococcus granulosus in Tasmania. Int J Parasitol 1991; 21: 959-961.





Abstract
Objective: To describe human hydatid disease in Tasmania since 1996, the year that the state was declared provisionally hydatid-free.
Design, setting and participants: Individuals with a new diagnosis or history of hydatid disease between January 1996 and July 2012 were identified through a number of sources including public health notifications, discharge coding from Tasmanian public hospitals, and the Royal Hobart Hospital pathology laboratory information system. Individuals were included if they fulfilled the case definition. Details regarding their diagnosis, management and risk factors were obtained by interview, review of medical notes, or both. The information was collected and analysed over a 3-month period from 30 July 2012 to 30 October 2012.
Main outcome measures: Patient demographics, site of infection, details of hydatid disease management and outcomes, time and place of likely hydatid acquisition, and public health notification.
Results: Fifty-one patients were identified, of whom 41 met the case definition. Twenty-five represented new diagnoses between 1996 and 2012. Median age was 71 years (range, 44–99 years). There were 21 women and 20 men. Thirty-eight patients had hepatic disease, five of whom had at least one other site involved. Four had extra-abdominal disease. Twenty-nine patients could be assessed for possible time and place of hydatid acquisition and all had significant risk factors for hydatid acquisition before 1980. Ten of the 25 patients diagnosed between 1996 and 2012 had been notified to the Tasmanian Department of Health and Human Services.
Conclusion: We found no evidence of transmission of hydatid disease to humans following the provisional declaration of eradication of hydatid disease.