Reproductive genetic carrier screening (RGCS) is a preventive health strategy performed to identify healthy couples and individuals who are at increased chance of having a child affected by a serious, childhood onset autosomal recessive or X‐linked genetic condition (Box 1).
When provided before conception or early in pregnancy, RGCS allows for increased chance couples to make informed reproductive choices for current or future pregnancies, and may reduce the chance of having an affected child or allow optimisation of health care requirements for an ongoing pregnancy.
Carrier screening programs for select autosomal recessive conditions with high prevalence in certain ethnic groups, such as Tay–Sachs disease in the Ashkenazi Jewish population, have been performed using non‐genetic blood parameters since the 1970s.1 Identification of carrier status using genetic testing was introduced in the 1990s, through targeted testing of genetic variants in genes associated with conditions such as cystic fibrosis.2
Over the past two decades, technologies including next generation sequencing have allowed for simultaneous analysis of thousands of genes with dramatic, continuous improvements in associated costs and turnaround time. This has enabled a shift from targeted testing of small numbers of single gene disorders to allow for the determination of an individual's carrier status for hundreds of diseases simultaneously. This has been broadly termed “expanded carrier screening”. The first expanded carrier screening platform became commercially available in 2009,3 followed by the release of international laboratory guidelines in 2013.4 A range of commercial expanded carrier screening panels analysing up to 1300 genes have subsequently become available to self‐funded individuals, allowing for the detection of carrier status of hundreds of autosomal recessive and X‐linked conditions.
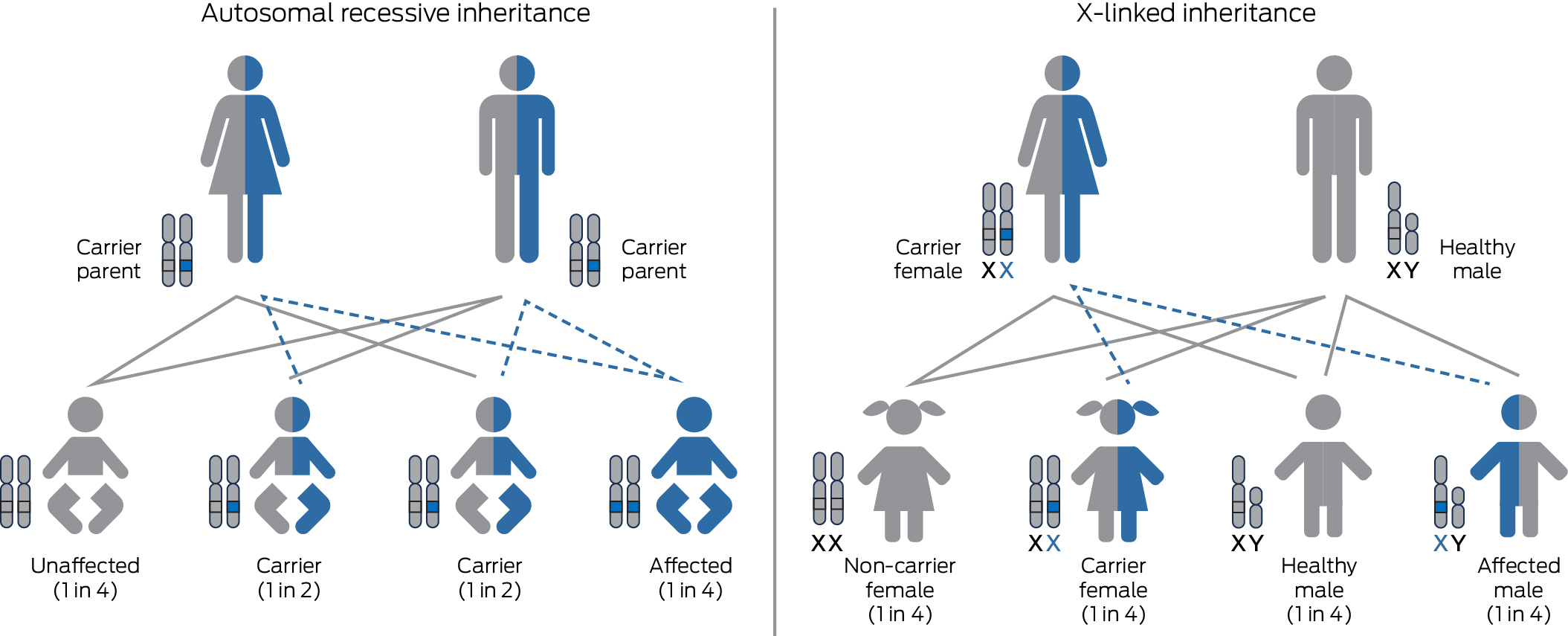
Since 2019, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists has recommended that information on RGCS be offered to all women early in pregnancy, or when planning a pregnancy.5 The impact of autosomal recessive genetic conditions has been found to be comparable to conditions that are already screened for in routine prenatal care, such as Down syndrome and neural tube defects.6,7 Each individual carries at least two recessive disease‐causing variants and 1–2% of all non‐consanguineous reproductive couples will be carriers for the same severe autosomal recessive disease or an X‐linked disease, and therefore have an increased chance (up to 1 in 4) of having an affected child.6,8
Despite its utility, the uptake of RGCS has been limited by many factors including the lack of awareness and understanding of both consumers and health practitioners, and the need to self‐fund the considerable cost of testing.9 Until recently, publicly funded genetic carrier testing was reserved for genetic relatives of an individual affected by a limited range of conditions. In 2020, the Medical Services Advisory Committee (MSAC) supported an application by the Royal College of Pathologists of Australasia for all women in early pregnancy or planning a pregnancy, and their respective partners where required, to be eligible for Medicare‐funded screening for three genetic conditions: cystic fibrosis, spinal muscular atrophy, and fragile X syndrome (commonly referred to as the three‐gene screen; Box 2). These item numbers became available on 1 November 2023. Simultaneously, MSAC approved Medicare Benefit Scheme (MBS) item numbers for individuals who identify as being of Ashkenazi Jewish descent to access RGCS for up to nine autosomal recessive conditions more commonly present in this population.
Based on the widespread community acceptance of other government‐funded screening tests in pregnancy (eg, first trimester biochemical screening) and pilot programs, it is reasonable to expect a high level of uptake of RGCS in Australia.
Why the three‐gene screen?
There is ongoing debate surrounding how many and which genes to include in RGCS panels.10,11,12 Criteria for appropriate genetic conditions include: a clear genotype–phenotype correlation; a severe phenotype that may affect reproductive decision making or one for which targeted treatment is available to improve health outcomes; a high prevalence of carriers in the screened population; a screening method with high sensitivity; and the availability of prenatal diagnosis and reproductive options.11,13
In the Australian population, cystic fibrosis is the most common life‐limiting autosomal recessive condition, spinal muscular atrophy is the most frequent genetic cause of infant mortality, and fragile X syndrome is the most common cause of X‐linked intellectual disability.7 As the first disease‐causing gene to be recognised, population‐based carrier screening for pathogenic CFTR variants has been under consideration since the late 1990s, and available in Australia since 2006.7,14,15,16 In 2012, Victorian Clinical Genetics Services offered the first Australian simultaneous carrier screening panel which included cystic fibrosis, together with spinal muscular atrophy and fragile X syndrome. A study of the first 12 000 of these tests established that about 1 in 20 Australian individuals are carriers for one or more of these conditions and 1 in 240 couples are at increased chance of having an affected child. Most carriers have no personal or family history of the condition.11
While collectively common, cystic fibrosis, spinal muscular atrophy and fragile X syndrome are distinct conditions with considerable differences (Box 2). For example, carrier status determination for each condition requires a different testing methodology, with spinal muscular atrophy and fragile X syndrome not routinely detectable on next generation sequencing. New targeted therapies are now available for cystic fibrosis and spinal muscular atrophy, which if instituted early in life may have dramatic impact on health outcomes. As a result, newborn screening for spinal muscular atrophy is now being introduced across Australia.17,18,19 Notably, no current curative treatment is available for any of the three conditions, all of which affect quality of life and/or life expectancy.
There are no provisions in the Medicare rebate for pre‐ or post‐test counselling
MSAC has assessed that pre‐test counselling can be adequately provided by health practitioners including obstetricians and general practitioners. However, there are implicit complexities in pre‐test counselling for RGCS that make it a potentially time consuming and resource intense process, including the fact that multiple RGCS providers are available. This implies a need for appropriate education of the health practitioner providing the counselling and/or access to appropriate consumer information resources. Most companies offering RGCS provide access to such resources, often online.
Pre‐test counselling for RGCS should be choice based. RGCS should only ever be offered as opt‐in and should allow for couples to make an informed choice about screening options. Individuals should be given the opportunity to prepare for a clinically significant result, which may lead to complex decision making in a current or future pregnancy. In addition, couples should be made aware of the limitations inherent in any form of screening test.
In some instances, findings may have implications for an individual's own health or that of their family members, and have the potential to limit the access to certain types of insurance.21,22 A pertinent example of complicated pre‐test counselling is the communication of the potential implications for a female being identified as a carrier of fragile X syndrome. While also conferring an increased chance of having a child with fragile X syndrome, carriers are at risk of developing fragile X tremor ataxia syndrome or fragile X‐associated premature ovarian insufficiency. Adequate counselling needs to address all possibilities.
Practitioners providing post‐test counselling for couples in the setting of an increased chance result will often be faced with an even higher level of complexity for numerous reasons. These include the implications and potential variability of the condition itself and the reproductive options available. Other issues that must be addressed are the potential ramifications for family members such as the prior children or siblings of prospective parents. A range of specialties will be involved, primarily clinical geneticists and genetic counsellors. Other services will include maternal fetal medicine, and other obstetric services offering reproductive options such as prenatal testing and in vitro fertilisation (IVF) with pre‐implantation genetic testing (PGT). Additionally, specialists involved in the care of affected children will require consultation.
Driven by the genomics revolution, Australian clinical genetics services (including both clinical geneticists and genetic counsellors) are in high demand, have long waitlists, and are under‐resourced to manage the surge of increased chance couples who will inevitably be unveiled by the new MBS item.
Identification of increased chance individuals or couples provides valuable access to reproductive options
In the pre‐pregnancy setting, reproductive options to reduce the risk of an affected pregnancy include: accessing IVF with PGT, or utilising prenatal testing (either chorionic villus sampling or amniocentesis); adoption or fostering; utilising donor egg, sperm or embryos with assisted reproductive technologies; or choosing not to have children.11,21 Some couples may choose not to access any of these options and accept the risk.
If, however, a couple is found to be at increased chance in an established pregnancy, their options are more limited. In this setting, families can process and gather information about a condition and may choose to test their pregnancy for the condition. Prenatal testing can relieve anxiety if the fetus is shown to be unaffected. If the fetus is shown to be affected by the condition, this allows discussion about targeted perinatal care, with anticipation of medical needs and outcomes after birth, or the potential choice of termination of the pregnancy.24
RGCS should be performed in the pre‐pregnancy setting where possible
In addition to being afforded a wider range of reproductive options, when RGCS is performed in the pre‐pregnancy setting, couples are allowed more time for appropriate pre‐test and post‐test counselling, and follow‐up testing where required.
The new MBS items for cystic fibrosis, spinal muscular atrophy and fragile X syndrome will allow sequential RGCS, where the female reproductive partner is tested first, and the reproductive partner is only offered testing following the identification of an autosomal recessive variant in the female. An alternative is couple‐based testing, where both reproductive partners are tested in parallel. Sequential testing is less expensive, as it avoids unnecessary testing in the male partner when the female is not a carrier. However, this can create additional pressure and stress in the setting of an established pregnancy, because each individual's RGCS, and prenatal testing where required, will take a matter of weeks to return a result. Consequently, a pregnancy may have reached an advanced gestation before the genetic status of the pregnancy is clarified.21,22 In the setting of an affected pregnancy, this potentially limits access to options such as termination of pregnancy.
This is just the beginning: Mackenzie's Mission and expanded carrier screening
Named in honour of Mackenzie Casella, who died of spinal muscular atrophy aged 7 months in 2017, Mackenzie's Mission was funded by the Australian Government Medical Research Future Fund as part of the Genomics Health Futures Mission. Recruitment of nearly 10 000 couples occurred between 2020 and 2022, and the results of this study are expected to be published imminently.
Mackenzie's Mission aimed to assess the acceptability and feasibility of an easily accessible expanded carrier screening program for Australian couples. After considerable work on mapping implementation needs and providing education to health practitioners, reproductive couples were recruited to be screened for over 1200 genes associated with about 750 serious, childhood‐onset genetic conditions. Recruited couples were provided with detailed, primarily online pre‐test education. All increased chance couples were urgently referred to local genetics services for result counselling, support, follow‐up, and access to prenatal testing where requested. Non‐pregnant increased chance couples were also offered access to one subsidised IVF cycle for PGT.23
Preliminary results from this study found that about 1 in 50 Australian reproductive couples will be identified as increased chance when expanded carrier screening is implemented.24 We expect that the community, health economic and clinical benefits of expanded carrier screening will inevitably lead to widespread uptake in the near future. However, this will be complex and will require considerable ongoing educational, counselling and clinical resources.
The MBS item supporting the three‐gene form of RGCS is a ground‐breaking moment in reproductive genetics that, for the first time in Australian history, offers funded large scale genetic screening to the general population. The workforce implications of the implementation of three‐gene RGCS are considerable. Management of the 1 in 240 increased chance couples will likely be undertaken by already stretched hospital‐based clinical genetics services, with flow‐on effects to other specialties. Management of individual carriers will not be possible within these services. Ongoing education of primary care health practitioners is essential at minimum to manage these demands, as most pre‐ and post‐test counselling will be delivered by general practitioners. However, management of families with an increased chance result, together with the inevitable evolution to widespread uptake of expanded carrier screening for hundreds of disorders beyond the three‐gene screen, will require much broader resourcing.
Carrier screening is a pertinent example of the complex, family‐based and evolving nature of genomic medicine. It highlights the need for government bodies and policy makers to prioritise the upskilling and education of non‐genetics professionals, while allocating resources to the already over‐stretched clinical and laboratory genetics services. Community‐based genetic counsellors would be ideally placed to support other health professionals in navigating RGCS, but there is currently no funding model to support this.
Box 2 – Summary of clinical and genetic features of cystic fibrosis, spinal muscular atrophy, and fragile X syndrome
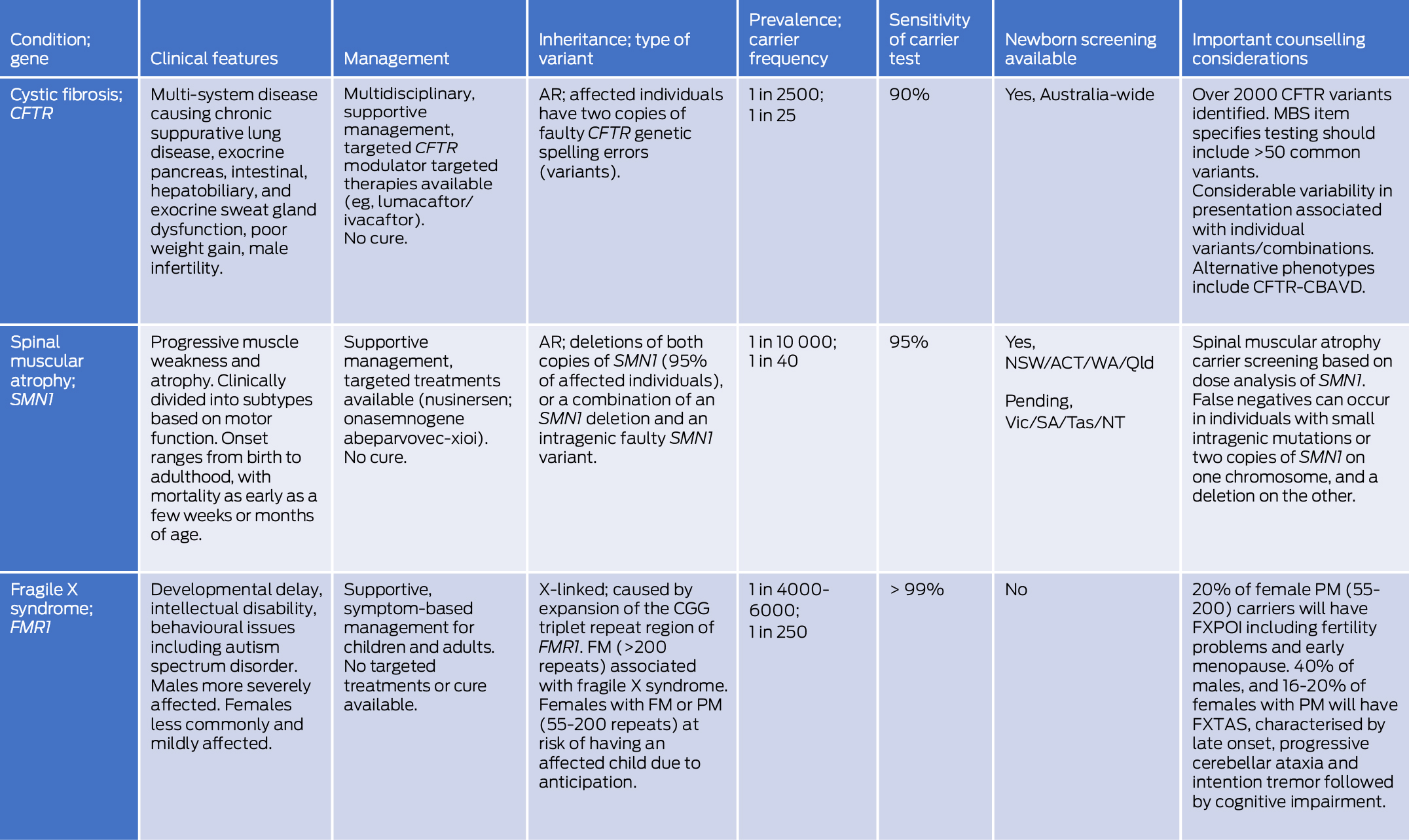
ACT = Australian Capital Territory; AR = autosomal recessive; CFTR = cystic fibrosis transmembrane conductance regulator; CFTR‐CBAVD = CFTR‐related isolated congenital bilateral absence of the vas deferens; FM = full mutation (> 200 CGG repeats); FMR1 = fragile X messenger ribonucleoprotein 1; FXPOI = fragile X‐associated primary ovarian insufficiency; FXTAS = fragile X‐associated tremor/ataxia syndrome; MBS = Medicare Benefits Schedule; NSW = New South Wales; NT = Northern Territory; PM = premutation (55–200 CGG repeats); Qld = Queensland; SA = South Australia; SMN1 = survival of motor neuron 1; Tas = Tasmania; Vic = Victoria; WA = Western Australia.
Provenance: Commissioned; externally peer reviewed.
- 1. Kaback MM, Nathan TJ, Greenwald S. Tay‐Sachs disease: heterozygote screening and prenatal diagnosis—US experience and world perspective. Prog Clin Biol Res 1977; 18: 13‐36.
- 2. Watson EK, Mayall E, Chapple J, et al. Screening for carriers of cystic fibrosis through primary health care services. BMJ 1991; 303: 504‐507.
- 3. Srinivasan BS, Evans EA, Flannick J, et al. A universal carrier test for the long tail of Mendelian disease. Reprod Biomed Online 2010; 21: 537‐551.
- 4. Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med 2013; 15: 482‐483.
- 5. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Genetic carrier screening. Mar 2019. https://ranzcog.edu.au/wp‐content/uploads/2022/05/Genetic‐carrier‐screeningC‐Obs‐63New‐March‐2019_1.pdf (viewed Nov 2023).
- 6. Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA 2016; 316: 734‐742.
- 7. Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genet Med 2018; 20: 513‐523.
- 8. Fridman H, Yntema HG, Mägi R, et al. The landscape of autosomal‐recessive pathogenic variants in European populations reveals phenotype‐specific effects. Am J Hum Genet 2021; 108: 608‐619.
- 9. Best S, Long J, Theodorou T, et al. Health practitioners’ perceptions of the barriers and enablers to the implementation of reproductive genetic carrier screening: a systematic review. Prenat Diagn 2021; 41: 708‐719.
- 10. Goldberg JD, Pierson S, Johansen Taber K. Expanded carrier screening: what conditions should we screen for? Prenat Diagn 2023; 43: 496‐505.
- 11. Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X‐linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021; 23: 1793‐1806.
- 12. Henneman L, Borry P, Chokoshvili D, et al. Responsible implementation of expanded carrier screening. Eur J Hum Genet 2016; 24: e1‐e12.
- 13. Committee Opinion No. 690 summary: Carrier screening in the age of genomic medicine. Obstet Gynecol 2017; 129: e35‐e40.
- 14. Grody WW, Cutting GR, Klinger KW, et al. Laboratory standards and guidelines for population‐based cystic fibrosis carrier screening. Genet Med 2001; 3: 149‐154.
- 15. Massie J, Petrou V, Forbes R, et al. Population‐based carrier screening for cystic fibrosis in Victoria: the first three years experience. Aust N Z J Obstet Gynaecol 2009; 49: 484‐489.
- 16. Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989; 245: 1073‐1080.
- 17. Savant A, Lyman B, Bojanowski C, Upadia J. Cystic fibrosis. In: Adam MP, Feldman J, Mirzaa GM, et al, editors. GeneReviews. Initial posting: 26 Mar 2001; last revision: 9 Mar 2023. https://www.ncbi.nlm.nih.gov/books/NBK1250/ (viewed Nov 2023).
- 18. Prior TW, Leach ME, Finanger E. Spinal muscular atrophy. In: Adam MP, Feldman J, Mirzaa GM, et al, editors. GeneReviews. Initial posting: 24 Feb 2000; last revision: 3 Dec 2020. https://www.ncbi.nlm.nih.gov/books/NBK1352/ (viewed Nov 2023).
- 19. D'Silva AM, Kariyawasam DST, Best S, et al. Integrating newborn screening for spinal muscular atrophy into health care systems: an Australian pilot programme. Dev Med Child Neurol 2022; 64: 625‐632.
- 20. Schofield D, Lee E, Parmar J, et al. Economic evaluation of population‐based, expanded reproductive carrier screening for genetic diseases in Australia. Genet Med 2023; 25: 100813.
- 21. Delatycki MB, Laing NG, Moore SJ, et al. Preconception and antenatal carrier screening for genetic conditions: The critical role of general practitioners. Aust J Gen Pract 2019; 48: 106‐110.
- 22. Sparks TN. Expanded carrier screening: counseling and considerations. Hum Genet 2020; 139: 1131‐1139.
- 23. Archibald AD, McClaren BJ, Caruana J, et al. The Australian Reproductive Genetic Carrier Screening Project (Mackenzie's Mission): design and implementation. J Pers Med 2022; 12: 1781.
- 24. Mackenzie's Mission. Outcomes of the Mackenzie's Mission study. https://www.mackenziesmission.org.au/outcomes/ (viewed Nov 2023).






Open access:
Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Jan Liebelt and Lara Fitzgerald are employed by Repromed, whose laboratory offers preconception RGCS. They also provide genetic counselling services.