Clinical record
A 16‐year‐old female patient with cystic fibrosis developed recurrent pleural effusions, facial plethora and prominent chest wall veins. She was homozygous for the ΔF508 mutation in the CFTR gene and was receiving elexacaftor/tezacaftor/ivacaftor cystic fibrosis transmembrane receptor modulator combination therapy. She had a left internal jugular vein (IJV) approach totally implantable venous access device (TIVAD, “portacath”) for central venous access. The TIVAD was used for frequent antibiotic lung “tune‐ups”: elective hospital admissions for intravenous antibiotics and chest physiotherapy to reduce bacterial lung infections. This TIVAD was inserted surgically five years previously, replacing a previous blocked left IJV TIVAD placed eight years ago. The current TIVAD could not be aspirated for use. A contrast‐enhanced computed tomography (CT) scan of the thorax showed occlusion of both brachiocephalic veins (BCVs) with patent superior vena cava (SVC). This is classified as Society of Interventional Radiology (SIR) type 3 pattern of obstruction with systemic venous return — a multisocietal consensus method of classifying the site and effect of central venous obstruction.1 A diagnosis of catheter‐related SVC syndrome (SVCS) was made. She was given warfarin on haematology advice (target international normalised ratio [INR], 2–3) to reduce the development of upper extremity deep vein thrombosis. Surgical removal of the TIVAD was unsuccessful, with the surgical team noting that the intravenous catheter was firmly adherent to the left IJV and BCV and could not be removed.
After multidisciplinary team meeting and discussion with the patient's family, she was referred to Interventional Radiology for catheter removal, stent grafting of the central venous occlusion and insertion of new central venous access. This approach aimed to treat her symptoms of central venous obstruction and restore central venous access for future treatments, given her expected long life expectancy.
Under general anaesthesia, central venography confirmed CT findings. The retained 15 cm of intravenous catheter was noted in the left BCV and IJV as well as large, well established vertebral and hemiazygos venous collaterals (Box 1, A).1 The retained catheter was removed intact using angioplasty and a snare device via the left neck. To optimise inline blood flow to the SVC, the decision was made to recanalise the right BCV only. This is technically easier than bilateral venous reconstruction, with reduced rate of significant procedural complications but equal SVCS symptom improvement. Covered stenting over the left central venous outflow does not cause contralateral upper limb swelling.2,3 Attempted wire recanalisation of the occluded right BCV from above and below was unsuccessful due to well developed collaterals and chronic fibrous obstruction. A large angioplasty balloon was placed via the right common femoral vein into the SVC. The inflated balloon was used as a target for sharp wire recanalisation from the right neck, re‐establishing continuity with the SVC. An 8 × 59 mm balloon expandable covered stent (off‐label usage; Viabahn VBX, WL Gore and Associates; Supporting Information and Box 1, B) was placed precisely from the patent origin of the right BCV to the patent SVC and dilated to 16 × 42 mm. Sixteen millimetres is an adult SVC diameter, in case of further patient growth, and allows good venous flow, but reduces the risk of intraprocedural venous rupture and pericardial tamponade associated with larger venous stents.3 Completion venography showed rapid inline flow with disappearance of collaterals (Box 1, C). A 6.6 French low profile single lumen TIVAD was inserted via the right IJV, with the line tip at the superior cavoatrial junction.4,5 At the end of the procedure, her anterior chest wall veins were no longer visible and facial plethora had improved. Her SVCS symptoms resolved within 24 hours, and she was discharged home ten days later after a planned cystic fibrosis tune‐up using her new TIVAD. She remains well more than one year after the procedure, with no return of the SVCS symptoms. She remains on warfarin (target INR, 2–3) as a precaution due to the remaining central venous catheter.6 She continues on regular clinical review for cystic fibrosis, with instructions to seek prompt medical advice if she develops any recurrence of the SVCS symptoms.
Discussion
SVCS is the result of thoracic systemic central vein obstruction, defined as pathological narrowing of the subclavian, intrathoracic IJVs, BCVs or SCV.1 Narrowing can affect any combination of central veins. About 60% of SVCS cases are caused by intrathoracic malignancy, typically primary lung cancer or lymphoma, but non‐malignant causes are increasingly common, predominantly due to previous central venous catheters or pacemaker leads.1,7,8 SVCS symptoms can range from minimal to life‐threatening (Box 2), depending on the location, extent of occlusion and acuity of onset — symptoms are worse with more proximal location, increasing stenosis, and rapid onset.1 Non‐malignant SVCS differs from malignancy‐associated SVCS in that a long life expectancy is expected with the former, thus requiring a long‐lasting treatment option.7,8
All types of central venous catheters can cause central venous stenosis, including peripherally inserted central catheters, particularly if the catheter tip is suboptimally positioned.9,10 Symptomatic SVCS requiring treatment is uncommon and asymptomatic central venous stenosis can be observed. Prophylactic anticoagulation is not recommended for preventing thrombosis in patients with central venous catheters.6
Although rarely life‐threatening, SVCS symptoms are distressing for the patient and it substantially complicates upper body vascular access, particularly if future organ transplantation or chronic renal replacement therapy is needed.5,9,10,11 Using oral instead of intravenous antibiotics with similar efficacy or use of leadless pacemakers without transvenous leads will reduce SVCS.5,9 If central venous access is required, it is important to use a catheter with the smallest diameter possible, for the shortest time, with the shortest intravenous course, and with careful catheter tip positioning.5,9,12 The superior cavoatrial junction is a safe catheter tip location, reproducibly identified on chest x‐ray as a point two vertebral body units below the carina (Box 3), where one vertebral body unit is one vertebral body and the superior adjacent vertebral disc.4,5 This is more inferior than commonly appreciated when looking at the superior aspect of the right heart border or right main bronchus on chest x‐ray.4
Treatment of catheter‐related SVCS is challenging, but attempts to re‐establish venous patency are encouraged rather than using another uninjured vein. Endovascular stenting is recommended as the first line treatment of catheter‐related SVCS over surgical reconstruction, due to similar rapid efficacy, but with substantially reduced periprocedural morbidity.8 Endovascular stenting does not preclude future surgical treatment.8 Covered stents have higher primary patency and slower rate of symptom return, compared with uncovered (“bare”) stents, which is important for patients with long life expectancies.7 However, both stent types require long term surveillance for SVCS symptom recurrence, which would prompt central venography with fluoroscopy or CT to assess stent patency.7,13 Stent stenoses can often be managed endovascularly with thrombectomy or further stent placement.7,13 The role of anticoagulation after endovascular treatment of SVCS is unclear, with some studies suggesting no benefit of anticoagulation after stenting.2,13 Avoiding the costs and complications of long term anticoagulation is useful in a patient group with a long life expectancy and often other significant medical comorbid conditions. However, anticoagulation use should be guided by individual patient factors and haematology advice.2,6,13
Lessons from practice
- Superior vena cava syndrome (SVCS) secondary to previous central venous catheters or pacemaker leads is increasing in incidence and is challenging to treat.
- If central venous access is required, using the smallest catheter possible, for the shortest time and with careful tip positioning can reduce the risk of developing central venous stenosis.
- Endovenous stenting is the first line treatment of malignant and non‐malignant SVCS, offering durable and rapid symptom relief.
- Covered stents have longer patency than uncovered stents for SVCS and may not require long term anticoagulation, which is useful for patients with non‐malignant SVCS who have a long life expectancy.
Box 1 – (A) Central venography performed via bilateral upper limb contrast injection with the patient's arms by her side, showing bilateral brachiocephalic vein (BCV) obstruction (stars).* (B) Recanalised right BCV containing undeployed covered stent (star).† (C) Central venogram after covered stent deployment (arrows) in the right BCV showing rapid inline flow to the right atrium and disappearance of venous collaterals (compare with panels A and B)
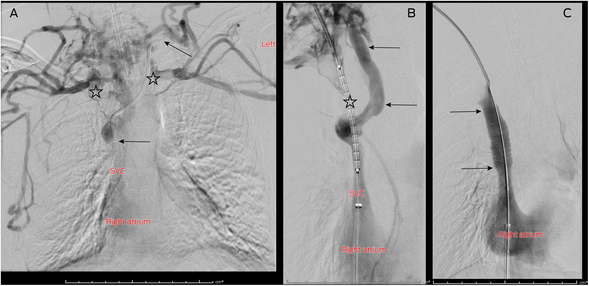
* Note the retained left BCV catheter (arrows). † Note the large paraspinal venous collateral bypassing the chronic right BCV occlusion (arrows).
Box 2 – Signs and symptoms of thoracic central venous occlusion and superior vena cava syndrome
|
Signs and symptoms |
Comment |
||||||||||||||
|
|
|||||||||||||||
|
Swelling |
Unilateral or bilateral, affecting head, face, neck, arms or breasts and can be objectively quantified with a tape measure |
||||||||||||||
|
Pain |
For example, headache secondary to venous hypertension |
||||||||||||||
|
Skin changes |
Red, blue or purple discolouration, non‐healing wounds in drained territory, distended neck or chest wall veins |
||||||||||||||
|
Respiratory distress |
Cough, pleural effusions, laryngeal oedema, or dyspnoea, particularly when lying flat |
||||||||||||||
|
Neurological symptoms |
Visual or auditory disturbances, dizziness, cognitive dysfunction, seizures, but other neurological causes should be excluded first before attributing to central venous occlusion |
||||||||||||||
|
|
|||||||||||||||
|
Adapted from Dolmatch et al.1 |
|||||||||||||||
Box 3 – Chest x‐ray following new right internal jugular vein totally implantable venous access device (TIVAD) insertion through new stent graft with resolution of bilateral pleural effusions*
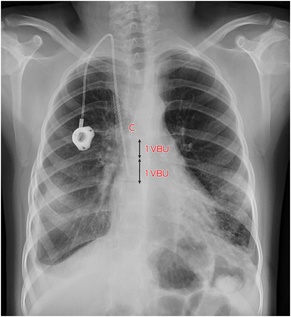
* Note the position of the catheter tip relative to the carina (C). One vertebral body unit (VBU = 1 vertebral body + 1 adjacent disc) is illustrated.
Provenance: Not commissioned; externally peer reviewed.
- 1. Dolmatch BL, Gurley JC, Baskin KM, et al. Society of Interventional Radiology Reporting Standards for Thoracic Central Vein Obstruction: Endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI). J Vasc Interv Radiol 2018; 29: 454‐460.
- 2. Cho Y, Gwon DI, Ko GY, et al. Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol 2014; 15: 87‐94.
- 3. Uberoi R. CIRSE quality assurance guidelines for superior vena cava stenting in malignant disease. Oxford: Cardiovascular and Interventional Radiological Society of Europe, 2015. https://www.cirse.org/wp‐content/uploads/2018/11/2015_‐SVC‐Stenting‐in‐Malignant‐Disease_Revision_Uberoi.pdf (viewed Jan 2024).
- 4. Baskin KM, Jimenez RM, Cahill AM, et al. Cavoatrial junction and central venous anatomy: implications for central venous access tip position. J Vasc Interv Radiol 2008; 19: 359‐365.
- 5. Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75 (Suppl): S1‐S164.
- 6. Rajasekhar A, Streiff MB. How I treat central venous access device‐related upper extremity deep vein thrombosis. Blood 2017; 129: 2727‐2736.
- 7. Haddad MM, Simmons B, McPhail IR, et al. Comparison of covered versus uncovered stents for benign superior vena cava (SVC) obstruction. Cardiovasc Intervent Radiol 2018; 41: 712‐717.
- 8. Rizvi AZ, Kalra M, Bjarnason H, et al. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg 2008; 47: 372‐380.
- 9. Garcilazo NHH, Hassanein M, Vachharajani TJ, Anvari E. Can I place a peripherally inserted central catheter in my patient with chronic kidney disease? Cleve Clin J Med 2021; 88: 431‐433.
- 10. Shin HS, Towbin AJ, Zhang B, et al. Venous thrombosis and stenosis after peripherally inserted central catheter placement in children. Pediatr Radiol 2017; 47: 1670‐1675.
- 11. Otani S, Westall GP, Levvey BJ, et al. Managing central venous obstruction in cystic fibrosis recipients — lung transplant considerations. J Cyst Fibros 2015; 14: 255‐261.
- 12. Sohail MA, Vachharajani TJ, Anvari E. Central venous catheters for hemodialysis — the myth and the evidence. Kidney Int Rep 2021; 6: 2958‐2968.
- 13. Haddad MM, Thompson SM, McPhail IR, et al. Is long‐term anticoagulation required after stent placement for benign superior vena cava syndrome? J Vasc Interv Radiol 2018; 29: 1741‐1747.






Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Patient consent:
The patient's parent gave written consent for publication.
No relevant disclosures.