The known: Chronic respiratory diseases are a major cause of poor health and mortality among Aboriginal and Torres Strait Islander people. Little is known about the prevalence of bronchiectasis among adult Indigenous Australians.
The new: The prevalence of bronchiectasis among adult Indigenous Australians in the Top End of the Northern Territory and the associated all‐cause mortality are alarmingly high. The prevalence is highest among people aged 40 years or more, and is generally higher among women than men and in rural and remote areas than in urban communities.
The implications: Characterising the epidemiology of bronchiectasis in Indigenous Australia is crucial for its accurate diagnosis and effective management, as well as for designing population‐level strategies for improving health outcomes.
Bronchiectasis is a chronic respiratory condition characterised radiologically by dilatation of the bronchial airways and clinically by chronic cough and sputum production.1 Bronchiectasis is an emerging global problem, and its prevalence is higher among people living with social disadvantage (including indigenous peoples in high income countries) or in low to middle income countries.2,3 The increase in the reported global prevalence of bronchiectasis may reflect a genuine rise or be related to greater recognition and diagnosis of what was once regarded an orphan disease.4 Its estimated prevalence in the United Kingdom is 566 cases per 100 000 women and 485 per 100 000 men;5 in the United States, the estimated prevalence is 1106 cases per 100 000 people over 65 years of age.6
For many indigenous peoples, the adult burden of chronic respiratory disease is great, particularly that of bronchiectasis, which is associated with poor health outcomes.7,8,9,10 Little has been reported about the prevalence of bronchiectasis in adult Aboriginal and Torres Strait Islander people (the Indigenous peoples of Australia).11 The first Australian Bronchiectasis Registry report (2019), based primarily on information from east coast tertiary centres, included data for only a small number of Indigenous people.12 The burden of bronchiectasis among Indigenous children in Central Australia, however, is great; in 2002, the estimated prevalence was 1470 cases per 100 000 children under 15 years of age,13 almost three times the United Kingdom adult rate. A small study found that the prevalence among adults in Central Australia was 103 cases per 10 000 Indigenous people, but only 1.01 per 10 000 non‐Indigenous people.14
Information about the prevalence and outcomes of bronchiectasis for indigenous children and adults in most countries is limited,11,15 despite the high morbidity and mortality associated with respiratory disorders in indigenous peoples.3,7,8,9,10,11,12,13,14,15 Understanding the epidemiology of bronchiectasis in Indigenous Australia — crucial for accurate diagnosis, effective management, and population‐level strategies for improving health outcomes —requires estimating its prevalence and its impact on morbidity in adults, particularly in the Top End of the Northern Territory, where the burden of respiratory disease is high.16,17,18 We therefore examined the prevalence of bronchiectasis among Aboriginal and Torres Strait Islander adults living in the Top End Health Service region of the NT during 2011–2020 (overall, by sex, and by remoteness of residence), as well as mortality among Indigenous adults with bronchiectasis.
Methods
The Top End Health Service region, which covers about 35% of the NT (475 338 km2),19 is divided into four health districts — Darwin Urban, Darwin Rural, East Arnhem, and Katherine — that roughly correspond to Australian Bureau of Statistics (ABS) statistical areas level 3 (SA3s) (Box 1). About 3.3% of people in Australia are Indigenous people; in the Northern Territory, they comprise 26–30% of the population, a larger proportion than in any other Australian state or territory.19
Study participants
Data for all Indigenous adults (18 years or older) in whom bronchiectasis was confirmed by chest computed tomography (CT) during 1 January 2011 – 31 December 2020 were included in our analysis. An initial password‐protected database comprised data for all people with bronchiectasis diagnoses included in our earlier studies.8,16,17,18 We then searched inpatient hospital electronic medical records for people diagnosed with CT‐confirmed bronchiectasis during 2011–2020 (International Classification of Diseases, tenth revision [ICD‐10] code J47) during inpatient admissions to hospitals in the Top End Health Service region (Royal Darwin Hospital, Katherine District Hospital, Gove District Hospital). Electronic medical records for individual patients were linked using their unique health reference numbers after confirming the chest CT‐confirmed bronchiectasis diagnosis with the reporting radiologist. The residential location of patients was based on the community or suburb name most recently recorded in their hospital electronic medical records.
Prevalence and mortality data modelling
The Top End Health Service population profile (ie, for the four relevant SA3s) was extracted from 2011 ABS census data using the ABS TableBuilder tool (https://www.abs.gov.au/statistics/microdata‐tablebuilder/tablebuilder); we included people aged 18–115 years who identified themselves in the census as Aboriginal, Torres Strait Islander, or both. We assumed that all included people with bronchiectasis had the disease in 2011. Age was defined as age at the 2011 census date (9 August 2011). We estimated the age‐standardised prevalence of bronchiectasis for each of the four Top End Health Service health districts, using the total Top End Health Service Indigenous population as the standard population, stratified and weighted as described in the Supporting Information, table 1.
We extracted demographic data (age, sex) for Indigenous people with bronchiectasis in the four Top End Health Service health districts, and for those in individual communities with ten or more active cases of bronchiectasis. If fewer than ten cases were recorded in a community, and cases were recorded in another community within 50 km, their numbers were combined; if the total was ten or more cases, the results for the two communities were aggregated. The number of people with bronchiectasis was then tabulated for the Top End Health Service area and by health district. All‐cause mortality (to 30 April 2023) was also assessed overall and by health district, as well as by community when possible. Mortality data were extracted from hospital information systems; death registry data linkage was not performed.
Statistical analysis
Data for continuous variables are summarised as medians with interquartile ranges (IQRs), categorical data as frequencies and proportions. We report prevalence per 1000 adults with 95% confidence intervals (CIs) and annual mortality rates with 95% CIs by health district and community. All analyses were conducted in STATA IC 15.
Ethics approval
This study was approved by the human research ethics committee of the Northern Territory Department of Health and Menzies School of Health Research (HREC 2019–3547), which waived the requirement for individual patient consent to data access.
Results
A total of 23 722 Indigenous adults lived in the Top End Health Service area in 2011. During 2011–2020, 459 people received chest CT‐confirmed bronchiectasis diagnoses. Their median age was 47.5 years (IQR, 39.9–56.8 years), 254 were women (55.3%) (Box 2), and 425 lived in areas classified as remote (93.0%). The estimated prevalence of bronchiectasis was 19.4 per 1000 residents (20.6 per 1000 women; 18.0 per 1000 men). Prevalence increased with age, peaking at 45.7 per 1000 people at age 50–59 years; in most age groups, it was slightly higher for women than men (Box 3).
Differences in prevalence by health district
At least 50% of people with bronchiectasis were women in all health district except East Arnhem (42%). The median age of people with bronchiectasis was higher in the Darwin Urban district (56.9 years; IQR, 48.1–68.3 years) than in the other three health districts (each under 50 years; Box 4).
The age‐adjusted prevalence of bronchiectasis was 5.0 (95% CI, 1.4–8.5) cases per 1000 people in Darwin Urban, 18 (95% CI, 10–26) cases per 1000 people in Katherine, 20 (95% CI, 12–28) cases per 1000 people in East Arnhem, and 36 (95% CI, 26–46) cases per 1000 people in Darwin Rural (Box 5). The age‐adjusted prevalence was similar for women and men in all health districts except Darwin Rural (44 [95% CI, 28–59] cases per 1000 women; 28 [95% CI, 16–41] cases per 1000 men). Its prevalence was highest among people aged 50–59 years in the Darwin Rural district for both women (94 per 1000) and men (78 per 1000; Box 6).
Crude prevalence by community ranged from 15 to 98 cases per 1000 people; the proportions of cases by sex differed between communities (Box 7).
Mortality
By 30 April 2023, 195 people with bronchiectasis had died (42.5%), at a median age of 60.3 years (IQR, 50.3–68.9 years) and after median follow‐up of 11.7 years (IQR, 8.1–11.7 years). The proportion of people who had died was smallest in East Arnhem (33 of 103 people, 32%) and largest in Darwin Urban (21 of 34, 62%). The mean annual mortality rate was similarly highest in the Darwin Urban district (7.5%; 95% CI, 0–17%) (Box 8); the mortality rate varied markedly between years and health districts (Box 9). The median age at death was about ten years higher in the Darwin Urban district (67.8 years; IQR, 61.2–79.5 years) than in the other three health districts (Darwin Rural: 59.8 [IQR, 49.7–69.2] years; East Arnhem: 58.5 [IQR, 51.4–65.0] years; Katherine: 58.2 [IQR, 48.6–68.2] years).
Discussion
We report the first investigation in the NT Top End of the prevalence of bronchiectasis among Aboriginal and Torres Strait Islander adults and of mortality among Indigenous adults with CT‐confirmed bronchiectasis. The estimated prevalence of bronchiectasis in the Top End Health Service region in 2011 was 19.4 per 1000 Indigenous adults, four times the estimated prevalence in the United Kingdom at about the same time.5 Its prevalence was slightly higher among women than men, but with considerable variation by health district. All‐cause mortality was high among people with CT‐confirmed bronchiectasis (42% to April 2023).
In our study, most adults with bronchiectasis were aged 40–59 years (263 of 459, 57%; median, 47.5 years; IQR, 39.9–56.8 years), much younger than reported for non‐Indigenous Australian adults (mean, 59.4 years; standard deviation, 17.5 years).9 The prevalence of bronchiectasis was high among Australian Indigenous children twenty years ago,7,13 and it is likely that many people in our study developed the disorder during childhood rather than as adults.20,21 The actual prevalence may therefore be higher than we found, and early development of bronchiectasis may also be a reason for the lower median age in our study. Another interesting feature was the variation in bronchiectasis prevalence between Top End Health Service districts and between communities. The prevalence may actually be higher in certain health districts, but the differences could be related to social factors such as access to specialist health care, including access to chest CT for confirming the diagnosis of bronchiectasis.
All‐cause mortality among Indigenous adults with bronchiectasis during the follow‐up period was 42% (195 of 459), and the median age at death was low (60.3 years; IQR, 50.3–68.9 years). We could not ascertain causes of death in our study, or indeed whether they were related to a respiratory condition. However, multimorbidity is highly prevalent among Indigenous people in the Northern Territory, including cardiovascular, chronic kidney disease, and complex and advanced respiratory disorders.8,16,17,18 Mortality rates among people with bronchiectasis are high.9,10,11 Moreover, other respiratory conditions, such as chronic obstructive pulmonary disease, together with a history of smoking and poorer lung function parameters, increase the risk of death.22
As the combination of multimorbidity and bronchiectasis may underlie the high overall mortality we found, it is imperative that the total respiratory health burden among adult Indigenous people be reduced. Further, targeted efforts are needed to overcome intrinsic and extrinsic barriers that impair access to specialist health care, and may account for marked differences between Indigenous and non‐Indigenous people in bronchiectasis prevalence, including social disadvantage (eg, household overcrowding, economic disadvantage), indoor air pollution, recurrent respiratory infections, and geographic isolation.11 Moreover, improving cultural sensitivity, education and awareness, early detection and diagnosis, treatment adherence, multidisciplinary care, preventing infections, smoking cessation, pulmonary rehabilitation, research and data collection, telehealth and telemedicine, and community involvement are all critical.
Despite reports since 1958 about the bronchiectasis burden among indigenous peoples,23 the low number of subsequent studies mean that our knowledge of bronchiectasis in adult indigenous people remains limited. Following our study in adult Indigenous Australians, we recommend:
- investigating the social determinants that contribute to higher bronchiectasis prevalence among Indigenous than non‐Indigenous Australians;
- determining the demographic and clinical determinants of sex‐related differences in its prevalence;
- identifying other medical conditions that increase morbidity among Indigenous people with bronchiectasis;
- collecting prospective clinical and chest radiology data to establish diagnostic and severity classification criteria specific to Indigenous people;
- examining the association of microbiological findings with rates of hospital admission and mortality;
- exploring differences in the regional prevalence of bronchiectasis;
- improving the transition from child to adult respiratory services for ongoing care of Indigenous people with bronchiectasis;
- developing and implementing culturally appropriate diagnostic and therapeutic interventions for Indigenous people, including chest physiotherapy services that meet the needs of people in the Top End of the NT; and
- screening Indigenous people to identify bronchiectasis as early as possible to facilitate interventions that reduce morbidity and mortality.
These strategies, needed to close the health gap between Indigenous and non‐Indigenous adults in Australia,24 will require collaborative partnerships between primary health care workers, as well as the leadership and involvement of Aboriginal health workers and practitioners and Aboriginal health organisations.
Limitations
Our findings specifically pertain to Indigenous people in the Top End Health Service region of the NT. Further, data for non‐Indigenous people were not available for direct comparisons, and the prevalence of bronchiectasis in this region cannot be generalised to all Indigenous people in the NT or across Australia. A prospective study of bronchiectasis among both Indigenous and non‐Indigenous adults in the NT would better characterise its epidemiology and the relative disease burden among Indigenous adults.
Further, we included data only for people with chest CT‐confirmed bronchiectasis diagnosed during 2011–2020. We will have excluded people for whom only clinical or X‐ray evidence of bronchiectasis was available because access to radiology in remote Australian Indigenous communities is limited, and some people with CT‐confirmed bronchiectasis may have been diagnosed outside our study period. Consequently, we may have underestimated the burden of bronchiectasis among Indigenous people in the Top End of the NT.
Conclusion
The bronchiectasis disease burden among Indigenous adults in the NT Top End is high, with differences in its prevalence by health district, as is all‐cause mortality among adults with bronchiectasis. Our study provides an initial insight into the heavy burden of bronchiectasis in our region, and our findings indicate the importance of strategies for improving health management and outcomes across the life course of Indigenous people, with the ultimate goal of closing the health gap between Indigenous and non‐Indigenous Australians.
Box 1 – The four health districts in the Top End Health Service region, with total numbers of Indigenous adults and of Indigenous adult women (in parentheses), 2011, by health district*
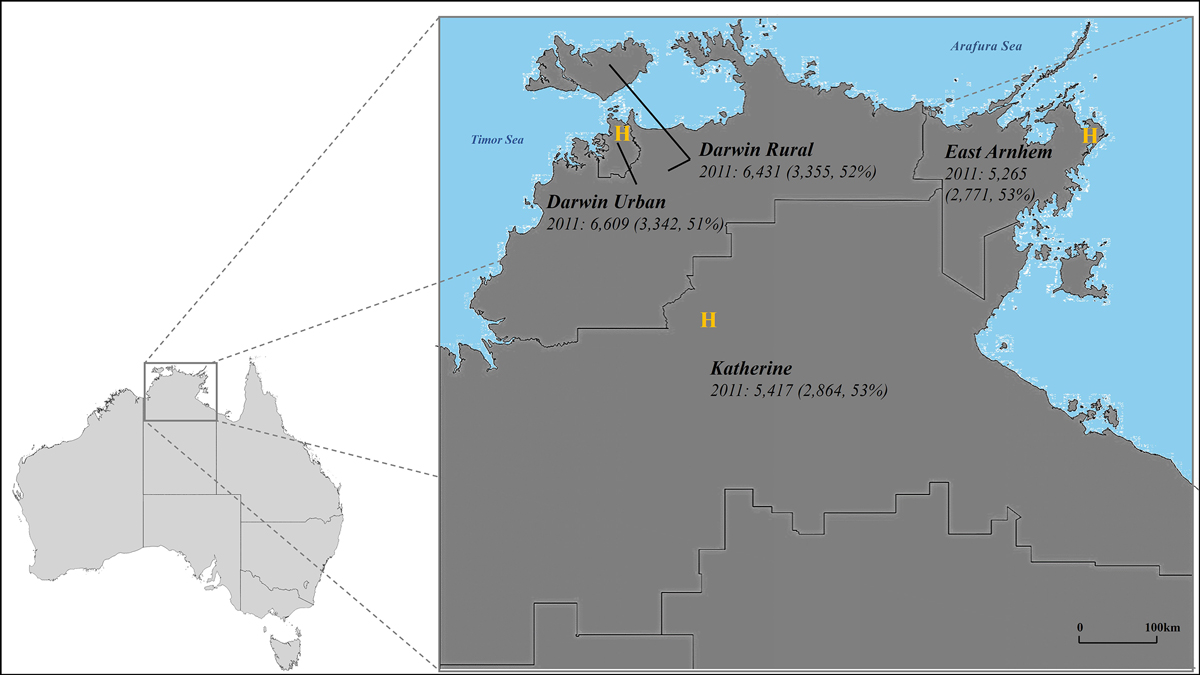
H = hospital.* Source: Australian Bureau of Statistics 2011 census data (https://www.abs.gov.au/statistics/microdata‐tablebuilder/tablebuilder). Estimated total number of Indigenous adults in the Top End, 2011: 23 722, including 12 322 women (52%).
Box 2 – Characteristics of Indigenous people with chest CT‐confirmed bronchiectasis in the Top End Health Service region, 2011–2020
|
Characteristic |
All people |
Women |
Men |
||||||||||||
|
|
|||||||||||||||
|
Number of people |
459 |
254 |
205 |
||||||||||||
|
Age (years), median (IQR) |
47.5 (39.9–56.8) |
47.7 (39.5–57.1) |
47.3 (40.2–56.2) |
||||||||||||
|
Age group (years) |
|
|
|
||||||||||||
|
18–29 |
35 (8%) |
21 (8%) |
14 (7%) |
||||||||||||
|
30–39 |
81 (18%) |
46 (18%) |
35 (17%) |
||||||||||||
|
40–49 |
145 (32%) |
74 (29%) |
71 (35%) |
||||||||||||
|
50–59 |
124 (27%) |
68 (27%) |
56 (27%) |
||||||||||||
|
60 or older |
74 (16%) |
45 (18%) |
29 (14%) |
||||||||||||
|
Health district |
|
|
|
||||||||||||
|
Darwin Urban |
34 (7%) |
18 (7%) |
16 (8%) |
||||||||||||
|
Darwin Rural |
226 (49%) |
140 (55%) |
86 (42%) |
||||||||||||
|
East Arnhem |
102 (22%) |
43 (17%) |
59 (29%) |
||||||||||||
|
Katherine |
97 (21%) |
53 (21%) |
44 (21%) |
||||||||||||
|
Deaths (to 30 April 2023) |
195 (42%) |
99 (39%) |
96 (47%) |
||||||||||||
|
Age at death (years), median (IQR) |
60.3 (50.3–68.9) |
60.9 (50.8–70.6) |
59.4 (49.5–66.9) |
||||||||||||
|
|
|||||||||||||||
|
CT = computed tomography; IQR = interquartile range. |
|||||||||||||||
Box 3 – Prevalence of CT‐confirmed bronchiectasis among Indigenous adults in the Top End Health Service region, 2011–2020, by age group and sex*
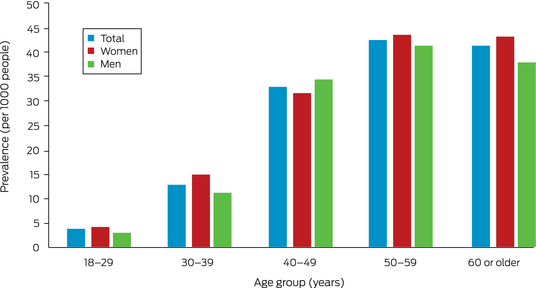
CT = computed tomography.* The data for this graph are included in the Supporting Information, table 2.
Box 4 – Characteristics of Indigenous people with chest CT‐confirmed bronchiectasis in the Top End Health Service region, 2011–2020, by health district
|
|
Health district |
||||||||||||||
|
Characteristic |
Darwin Urban |
Darwin Rural |
East Arnhem |
Katherine |
|||||||||||
|
|
|||||||||||||||
|
Number of people |
34 |
226 |
102 |
97 |
|||||||||||
|
Age (years), median (IQR) |
57.0 (48.1–68.3) |
46.7 (39.3–56.4) |
47.1 (40.5–55.2) |
48.1 (39.5–56.2) |
|||||||||||
|
Age group (years) |
|
|
|
|
|||||||||||
|
18–29 |
1 (3%) |
15 (7%) |
7 (7%) |
12 (12%) |
|||||||||||
|
30–39 |
2 (6%) |
47 (21%) |
17 (17%) |
15 (16%) |
|||||||||||
|
40–49 |
9 (26%) |
72 (32%) |
35 (34%) |
29 (30%) |
|||||||||||
|
50–59 |
10 (29%) |
55 (24%) |
35 (34%) |
24 (25%) |
|||||||||||
|
60 or older |
12 (35%) |
37 (16%) |
8 (8%) |
17 (18%) |
|||||||||||
|
Sex (women) |
18 (53%) |
140 (62%) |
43 (42%) |
53 (55%) |
|||||||||||
|
Deaths (to 30 April 2023) |
21 (62%) |
97 (43%) |
33 (32%) |
44 (45%) |
|||||||||||
|
Age at death (years), median (IQR) |
67.8 (61.1–79.5) |
59.8 (49.7–69.2) |
58.5 (51.4–65.0) |
58.2 (48.6–68.2) |
|||||||||||
|
|
|||||||||||||||
|
CT = computed tomography; IQR = interquartile range. |
|||||||||||||||
Box 5 – Prevalence of CT‐confirmed bronchiectasis per 1000 people (with 95% confidence intervals) among Indigenous adults in the Top End Health Service region, 2011–2020, by health district
|
|
Crude prevalence |
Age‐adjusted prevalence |
|||||||||||||
|
Health district |
All people |
Women |
Men |
All people |
Women |
Men |
|||||||||
|
|
|||||||||||||||
|
Darwin Urban |
5.1 (3.4–6.9) |
5.4 (2.9–7.9) |
4.9 (2.5–7.3) |
5.0 (1.4–8.5) |
5.0 (0–10) |
5.0 (0.3–9.8) |
|||||||||
|
Darwin Rural |
35 (31–40) |
42 (35–49) |
28 (22–34) |
36 (26–46) |
44 (28–59) |
28 (16–41) |
|||||||||
|
East Arnhem |
19 (16–23) |
16 (11–20) |
24 (18–30) |
20 (12–28) |
16 (6–26) |
24 (11–37) |
|||||||||
|
Katherine |
18 (14–21) |
18 (14–23) |
17 (12–22) |
18 (10–26) |
19 (7.6–30) |
17 (6.4–28) |
|||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 6 – Age‐adjusted prevalence of CT‐confirmed bronchiectasis among Indigenous adults in the Top End Health Service region, 2011–2020, by health district, age group, and sex
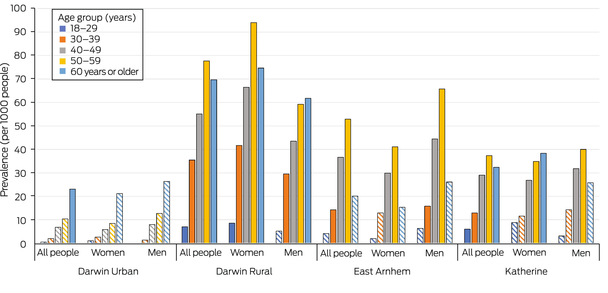
CT = computed tomography.* Striped columns: categories with fewer than ten cases of bronchiectasis recorded. The data for this graph are included in the Supporting Information, table 3.
Box 7 – Crude prevalence of CT‐confirmed bronchiectasis and mean annual mortality rate* (per 1000 people, with 95% confidence intervals) among Indigenous adults in the Top End Health Service region in communities with at least ten cases, 2011–2020, by community†
|
Community |
All people |
Women |
Men |
||||||||||||
|
|
|||||||||||||||
|
A: Cases (deaths) |
20 (8) |
12 (4) |
< 10 |
||||||||||||
|
Crude prevalence |
87 (51–124) |
99 (46–152) |
— |
||||||||||||
|
Mortality rate |
4 (0–11) |
4 (0–10) |
— |
||||||||||||
|
B: Cases (deaths) |
23 (11) |
< 10 |
14 (7) |
||||||||||||
|
Crude prevalence |
90 (55–125) |
— |
111 (56–166) |
||||||||||||
|
Mortality rate |
6 (0–14) |
— |
6 (0–13) |
||||||||||||
|
C: Cases (deaths) |
82 (34) |
54 (19) |
28 (15) |
||||||||||||
|
Crude prevalence |
98 (78–119) |
126 (94–157) |
70 (45–94) |
||||||||||||
|
Mortality rate |
4 (0–9) |
4 (0–8) |
6 (0–14) |
||||||||||||
|
D: Cases (deaths) |
16 (9) |
12 (7) |
< 10 |
||||||||||||
|
Crude prevalence |
26 (13–38) |
38 (17–59) |
— |
||||||||||||
|
Mortality rate |
7 (0–18) |
7 (0–19) |
— |
||||||||||||
|
E: Cases (deaths) |
41 (19) |
24 (11) |
17 (8) |
||||||||||||
|
Crude prevalence |
29 (20–38) |
34 (20–47) |
25 (13–36) |
||||||||||||
|
Mortality rate |
5 (0–12) |
5 (0–11) |
5 (0–14) |
||||||||||||
|
F: Cases (deaths) |
17 (6) |
11 (5) |
< 10 |
||||||||||||
|
Crude prevalence |
28 (15–40) |
34 (14–54) |
— |
||||||||||||
|
Mortality rate |
4 (0–10) |
5 (0–15) |
— |
||||||||||||
|
G: Cases (deaths) |
15 (4) |
< 10 |
< 10 |
||||||||||||
|
Crude prevalence |
23 (12–35) |
— |
— |
||||||||||||
|
Mortality rate |
3 (0–6) |
— |
— |
||||||||||||
|
H: Cases (deaths) |
16 (3) |
< 10 |
12 (3) |
||||||||||||
|
Crude prevalence |
72 (38–106) |
— |
129 (61–197) |
||||||||||||
|
Mortality rate |
2 (0–5) |
— |
2 (0–7) |
||||||||||||
|
I: Cases (deaths) |
28 (12) |
10 (3) |
18 (9) |
||||||||||||
|
Crude prevalence |
28 (18–39) |
19 (7–31) |
39 (21–56) |
||||||||||||
|
Mortality rate |
5 (0–12) |
3 (0–9) |
6 (0–16) |
||||||||||||
|
J: Cases (deaths) |
12 (7) |
< 10 |
< 10 |
||||||||||||
|
Crude prevalence |
28 (13–44) |
— |
— |
||||||||||||
|
Mortality rate |
7 (0–20) |
— |
— |
||||||||||||
|
K: Cases (deaths) |
12 (4) |
< 10 |
< 10 |
||||||||||||
|
Crude prevalence |
22 (10–34) |
— |
— |
||||||||||||
|
Mortality rate |
4 (0–10) |
— |
— |
||||||||||||
|
L: Cases (deaths) |
22 (10) |
12 (6) |
10 (4) |
||||||||||||
|
Crude prevalence |
15 (9–22) |
15 (7–24) |
15 (6–25) |
||||||||||||
|
Mortality rate |
5 (0–12) |
6 (0–12) |
4 (0–10) |
||||||||||||
|
M: Cases (deaths) |
14 (2) |
< 10 |
< 10 |
||||||||||||
|
Crude prevalence |
45 (22–68) |
— |
— |
||||||||||||
|
Mortality rate |
1 (0–4) |
— |
— |
||||||||||||
|
|
|||||||||||||||
|
CT = computed tomography. * Deaths to 30 April 2023. † Each capital letter refers to a community or cluster of nearby communities. |
|||||||||||||||
Box 8 – Mean annual mortality rate among Indigenous adults in the Top End Health Service region with CT‐confirmed bronchiectasis, 2011–2020, by health district and sex
|
Health district |
All people |
Women |
Men |
||||||||||||
|
|
|||||||||||||||
|
Darwin Urban |
|
|
|
||||||||||||
|
Cases (deaths*) |
34 (21) |
18 (9) |
14 (12) |
||||||||||||
|
Mean annual mortality rate (95% CI) |
7.5% (0–17%) |
5.3% (0–13%) |
10% (0–23%) |
||||||||||||
|
Darwin Rural |
|
|
|
||||||||||||
|
Cases (deaths*) |
226 (97) |
140 (54) |
86 (43) |
||||||||||||
|
Mean annual mortality rate (95% CI) |
4.5% (1.7–7.4%) |
3.9% (0.7–7.1%) |
5.5% (0.4–10%) |
||||||||||||
|
East Arnhem |
|
|
|
||||||||||||
|
Cases (deaths*) |
102 (33) |
43 (11) |
59 (22) |
||||||||||||
|
Mean annual mortality rate (95% CI) |
3.2% (0.1–6.2%) |
2.4% (0–5.9%) |
3.7% (0–8.0%) |
||||||||||||
|
Katherine |
|
|
|
||||||||||||
|
Cases (deaths*) |
97 (44) |
53 (25) |
44 (19) |
||||||||||||
|
Mean annual mortality rate (95% CI) |
4.9% (0.4–9.4%) |
5.1% (0–11%) |
4.6% (0–11%) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; CT = computed tomography. * Deaths to 30 April 2023. |
|||||||||||||||
Box 9 – Number of deaths and mortality rate* among Indigenous adults in the Top End Health Service region with CT‐confirmed bronchiectasis, 2011–2023, by health district and year
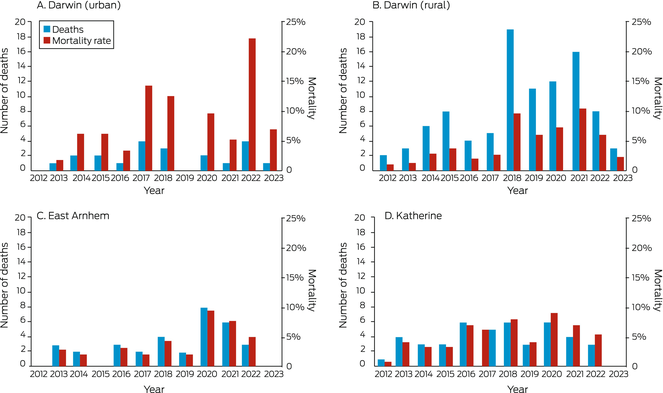
* Number of deaths per population at risk. No deaths were recorded in 2011; follow‐up ended at 30 April 2023 (only four months’ data for 2023). Initial populations (2011) were Darwin Urban, 34; Darwin Rural, 226; East Arnhem, 102; Katherine, 97. The data for this graph are included in the Supporting Information, table 4.
Received 24 June 2023, accepted 9 October 2023
- Claire Gibbs1,2
- Timothy Howarth3,4
- Adriana Ticoalu5
- Winnie Chen2,6
- Payi L Ford5
- Asanga Abeyaratne6
- Lata Jayaram7,8
- Gabrielle McCallum6
- Subash S Heraganahally1,2
- 1 Royal Darwin Hospital, Darwin, NT
- 2 Flinders University, Darwin, NT
- 3 Charles Darwin University, Darwin, NT
- 4 University of Eastern Finland, Kuopio, Finland
- 5 Northern Institute, Charles Darwin University, Darwin, NT
- 6 Menzies School of Health Research, Darwin, NT
- 7 Western Health, Melbourne, VIC
- 8 The University of Melbourne, Melbourne, VIC
Open access:
Open access publishing facilitated by Flinders University, as part of the Wiley ‐ Flinders University agreement via the Council of Australian University Librarians.
This study was supported by the Thoracic Society of Australia and New Zealand (TSANZ) Robert Pierce Grant‐In‐Aid for Indigenous Lung Health. The TSANZ did not have any role in the study design, data collection, analysis, or interpretation. We thank the TSANZ research grant assessment committee members for recognising this investigation as a priority for reducing the bronchiectasis disease burden among the adult Indigenous Australians.
No relevant disclosures.
- 1. King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis 2009; 4: 411–419.
- 2. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890.
- 3. de Boer S, Lewis CA, Fergusson W, et al. Ethnicity, socioeconomic status and the severity and course of non‐cystic fibrosis bronchiectasis. Intern Med J 2018; 48: 845–850.
- 4. Imam JS, Duarte AG. Non‐CF bronchiectasis: orphan disease no longer. Respir Med 2020; 166: 105940.
- 5. Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. Eur Respir J 2016; 47: 186–193.
- 6. Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest 2012; 142: 432–439.
- 7. McCallum GB, Binks MJ. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front Pediatr 2017; 5: 27.
- 8. Mehra S, Chang AB, Lam CK, et al. Bronchiectasis among Australian Aboriginal and non‐Aboriginal patients in the regional and remote population of the Northern Territory of Australia. Rural Remote Health 2021; 21: 6390.
- 9. Blackall SR, Hong JB, King P, et al. Bronchiectasis in indigenous and non‐indigenous residents of Australia and New Zealand. Respirology 2018; 23: 743–749.
- 10. Roberts ME, Lowndes L, Milne DG, Wong CA. Socioeconomic deprivation, readmissions, mortality and acute exacerbations of bronchiectasis. Intern Med J 2012; 42: e129‐e136.
- 11. Howarth T, Heraganahally SS, Heraganahally SS. Bronchiectasis among adult First Nations indigenous people: a scoping review. Curr Respir Med Rev 2023; 19: 36–51.
- 12. Visser SK, Bye PTP, Fox GJ, et al. Australian adults with bronchiectasis: the first report from the Australian Bronchiectasis Registry. Respir Med 2019; 155: 97–103.
- 13. Chang AB, Grimwood K, Mulholland EK, Torzillo PJ; Working Group on Indigenous Paediatric Respiratory Health. Bronchiectasis in Indigenous children in remote Australian communities. Med J Aust 2002; 177: 200–204. https://www.mja.com.au/journal/2002/177/4/bronchiectasis‐indigenous‐children‐remote‐australian‐communities
- 14. Einsiedel L, Fernandes L, Spelman T, et al. Bronchiectasis is associated with human T‐lymphotropic virus 1 infection in an Indigenous Australian population. Clin Infect Dis 2012; 54: 43–50.
- 15. Munro K, Singleton RJ, Edwards EA, et al. Burden of bronchiectasis in indigenous peoples: how can it be improved? Curr Pediatr Rev 2009; 5: 198–206.
- 16. Kruavit A, Fox M, Pearson R, Heraganahally S. Chronic respiratory disease in the regional and remote population of the Northern Territory Top End: a perspective from the specialist respiratory outreach service. Aust J Rural Health 2017; 25: 275–284.
- 17. Heraganahally SS, Wasgewatta SL, McNamara K, et al. Chronic obstructive pulmonary disease in Aboriginal patients of the Northern Territory of Australia: a landscape perspective. Int J Chron Obstruct Pulmon Dis 2019; 14: 2205–2217.
- 18. Heraganahally SS, Timothy TP, Sorger L. Chest computed tomography findings among adult Indigenous Australians in the Northern Territory of Australia. J Med Imaging Radiat Oncol 2022; 66: 337–344.
- 19. Australian Bureau of Statistics. Northern Territory: Aboriginal and Torres Strait Islander population summary. 1 July 2022. https://www.abs.gov.au/articles/northern‐territory‐aboriginal‐and‐torres‐strait‐islander‐population‐summary (viewed Mar 2023).
- 20. Schutz KL, Fancourt N, Chang AB, et al. Transition of pediatric patients with bronchiectasis to adult medical care in the Northern Territory: a retrospective chart audit. Front Pediatr 2023; 11: 1184303.
- 21. Sibanda D, Singleton R, Clark J, et al. Adult outcomes of childhood bronchiectasis. Int J Circumpolar Health 2020; 79: 1731059.
- 22. Choi H, Yang B, Kim YJ, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep 2021; 11: 7126.
- 23. Hinds JR. Bronchiectasis in the Maori. N Z Med J 1958; 57: 328–332.
- 24. Milroy T, Bandler LG. Closing the Gap: where to now? Med J Aust 2021; 214: 209–210. https://www.mja.com.au/journal/2021/214/5/closing‐gap‐where‐now






Abstract
Objectives: To assess the prevalence of bronchiectasis among Aboriginal and Torres Strait Islander (Indigenous) adults in the Top End of the Northern Territory, and mortality among Indigenous adults with bronchiectasis.
Study design: Retrospective cohort study.
Setting, participants: Aboriginal and Torres Strait Islander adults (18 years or older) living in the Top End Health Service region of the NT in whom bronchiectasis was confirmed by chest computed tomography (CT) during 1 January 2011 – 31 December 2020.
Main outcome measures: Prevalence of bronchiectasis, and all‐cause mortality among Indigenous adults with CT‐confirmed bronchiectasis — overall, by sex, and by health district — based on 2011 population numbers (census data).
Results: A total of 23 722 Indigenous adults lived in the Top End Health Service region in 2011; during 2011–2020, 459 people received chest CT‐confirmed diagnoses of bronchiectasis. Their median age was 47.5 years (interquartile range [IQR], 39.9–56.8 years), 254 were women (55.3%), and 425 lived in areas classified as remote (93.0%). The estimated prevalence of bronchiectasis was 19.4 per 1000 residents (20.6 per 1000 women; 18.0 per 1000 men). The age‐adjusted prevalence of bronchiectasis was 5.0 (95% CI, 1.4–8.5) cases per 1000 people in the Darwin Urban health area, and 18–36 cases per 1000 people in the three non‐urban health areas. By 30 April 2023, 195 people with bronchiectasis had died (42.5%), at a median age of 60.3 years (IQR, 50.3–68.9 years).
Conclusion: The prevalence of bronchiectasis burden among Indigenous adults in the Top End of the NT is high, but differed by health district, as is all‐cause mortality among adults with bronchiectasis. The socio‐demographic and other factors that contribute to the high prevalence of bronchiectasis among Indigenous Australians should be investigated so that interventions for reducing its burden can be developed.