Coronary artery disease (CAD) remains the leading cause of death in the world. Secondary prevention including antiplatelet, antihypertensive and lipid lowering medicines, as well as behavioural and lifestyle interventions are established treatments, and their implementation continues to be a global health system challenge.1 Yet even if well implemented, residual elevated risk remains for recurrent events, the nature of which may be changing in line with the changes seen to risk factor profiles of patients with CAD.2 Observational studies have shown that people with high inflammatory states and autoimmune conditions have a high prevalence of cardiovascular disease (CVD) and higher risk of repeat cardiovascular events, including mortality.3,4 Early evidence of this was observed in the Physicians’ Health Study when apparently healthy men with elevated C‐reactive protein (CRP) levels were at higher risk of cardiovascular events.5 Associations of high‐sensitivity CRP (hsCRP) and other elevated inflammatory markers such as tumour necrosis factor (TNF)‐α and interleukin (IL)‐6 with atherosclerosis and cardiovascular events, as demonstrated in multiple subsequent observational studies,6,7 all pointed to a mechanistic role for inflammation. Mechanistic studies have described the interplay between inflammatory and anti‐inflammatory components of the immune system which lead to plaque development and progression. Clinical studies suggest that CVD risk associated with inflammation is modifiable and, thus, an alternative new treatment target.6,8 Here, we discuss the case for targeting inflammation in the secondary prevention of CAD.
Chronic inflammation in atherosclerosis
Poor lipid metabolism results in stimulation of the immune system, with some epitopes of oxidised low‐density lipoprotein (LDL) triggering an innate and adaptive immune response.9 Endothelial injury, such as hypertension and oxidative stress, allows monocytes to enter the artery wall.9 LDL becomes entrapped in the intimal layer of the coronary artery, where monocytes transform into macrophages and engulf the LDL to form foamy macrophages.10 Macrophages also stimulate an adaptive immune response by activating T cells.9 T cells such as Th1 cells release pro‐inflammatory cytokines (eg, IL‐1β, TNF‐α and interferon [IFN]‐γ).9 Other immune cells such as neutrophils and mast cells also release pro‐inflammatory cytokines (eg, IFN‐γ, TNF‐α and IL‐6), which further recruit immune cells and inflammation.9 This all contributes to the chronic inflammatory process of atherosclerosis (Box 1). The high turnover of inflammatory cells leads to cell death and development of a necrotic core within the atherosclerotic plaque. There is also a counteracting anti‐inflammatory process. The exact role of protective T cells and reparative macrophage in atherosclerosis remains unclear with continued research interest, as they provide further alternative targets to atherosclerosis.1
Clinical trials
Although the mechanism of the immune system continues to be explored, therapeutic treatments that target inflammation have been reported. (Box 2 and Box 3). The first trial that raised attention on the potential use of existing anti‐inflammatory medication in CAD was the Australian‐ and Canadian‐led Low‐Dose Colchicine (LoDoCo) trial.11 This was a prospective, randomised, observer‐blinded, endpoint design among 532 patients with stable CAD, randomly assigned to colchicine 0.5 mg per day or no colchicine. At a mean follow‐up of 2.4 years, a primary outcome event (composite of acute coronary syndrome, out‐of‐hospital cardiac arrest, or non‐cardioembolic ischaemic stroke) occurred in 5% of the patients assigned to the colchicine group and in 16% of those assigned to the control group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.18–0.59; P < 0.001; number needed to treat, 11). The findings were surprising given the large effect size, though previous research had suggested plausible mechanisms. This included retrospective observations showing that continuous use of colchicine is associated with a lower than expected risk of myocardial infarction in patients with familial Mediterranean fever16 and gout, and colchicine is known to suppress neutrophils, a key contributor to plaque instability.
This trial was followed by the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS),6 in which canakinumab was administered to patients with a history of myocardial infarction and an elevated hsCRP. Canakinumab is a monoclonal antibody targeting IL‐1β, a pro‐inflammatory cytokine used in rheumatological diseases. The study compared three doses of canakinumab with placebo and showed a reduction in cardiovascular events (repeat myocardial infarction, stroke and cardiovascular death) in the population who received the 150 mg dose independent of their LDL‐cholesterol levels.6 Importantly, canakinumab did increase the risk of infection and sepsis compared with placebo, highlighting potential significant “off‐target” effects caused by chronic non‐specific inflammatory suppression.17
Two studies involving colchicine were released shortly after CANTOS: the Colchicine Cardiovascular Outcomes Trial (COLCOT)12 and the Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease 2 (LoDoCo2) trial.13 COLCOT randomly assigned 4745 patients to colchicine or placebo within 30 days of their myocardial infarction and showed a 23% relative risk reduction for cardiovascular death, stroke, repeat myocardial infarction or urgent hospitalisation for angina requiring revascularisation.12 LoDoCo2 randomly assigned 5522 patients with chronic coronary disease to colchicine or placebo and found a relative risk reduction of 31% for cardiovascular death, myocardial infarction, ischaemic stroke or ischaemia‐driven revascularisation over a median follow‐up of 28.6 months.13
Although CANTOS recruited patients with an elevated residual risk determined by measuring hsCRP, the colchicine trials did not. Primary and secondary endpoint rates in CANTOS were high, with cumulative incidences of more than 20% in five years in the placebo group despite controlled levels of LDL‐cholesterol, suggesting that hsCRP may be a potential measure of residual risk.6
The Cardiovascular Inflammation Reduction Trial (CIRT), a trial of low dose methotrexate, recruited patients with diabetes or the metabolic syndrome instead of hsCRP.14 Methotrexate did not reduce cardiovascular events or IL‐1β, IL‐6 or hsCRP compared with placebo. This is despite multiple observational studies demonstrating a reduction in CVD in patients with arthritis treated with methotrexate.18 Furthermore, there was a higher incidence of transaminitis, anaemia and infection. The CIRT trial highlights the importance of targeting appropriate populations with residual risk of CAD given the risks of immunosuppression. The mechanism of methotrexate continues to be researched but some studies suggest that although it reduces inflammatory cytokines in joints, it may increase inflammatory cytokines (IL‐1, IL‐6 and TNF) in macrophages.18
Smaller clinical studies have explored other treatments or combinations of treatments but have thus far been inconclusive. A recent pilot study of hydroxychloroquine showed promising lower IL‐6 levels in the treatment group compared with the placebo group with no reported serious adverse events.15 However, the trial only had a total of six cardiovascular events at 12 months and, thus, was inconclusive on clinical endpoints including adverse events. In addition, a trial of methotrexate and colchicine in stable CAD compared three groups: 24 patients assigned to low dose methotrexate and colchicine, 23 to colchicine and placebo, 24 to methotrexate and placebo, and 23 to placebo only. It found no difference at eight or 24 weeks in coronary endothelial measures.19
Clinical implications
A review pooling data from 11 594 patients from previously described trials (COLCOT, COPS [Colchicine in Patients with Acute Coronary Syndromes], LoDoCo, and LoDoCo2) reports significant reductions in cardiovascular events: 32% in the incidence of the composite of CVD mortality, myocardial infarction, ischaemic stroke and urgent coronary revascularisation; 38% for myocardial infarction; 62% for stroke; and 44% for urgent coronary revascularisation.16 This evidence has led to a class IIb recommendation for colchicine in the 2021 European Society of Cardiology guidelines for secondary prevention, particularly in patients with uncontrolled risk factors and recurrent events despite optimal medical therapy.20,21 Health Canada in 2021 also approved low dose colchicine in secondary prevention.22 Uncertainty remains about the long term benefits and safety of low dose colchicine; however, given widespread availability, low cost, and tolerability profile of a drug that has been used for years, there is reason for its incorporation into guidelines for the very high risk patient at least.
This differs from canakinumab with both the magnitude of its side‐effect profile and uncertainty in how to best target an at‐risk population. These issues need addressing before this or similar agents can be considered to have clinical utility. Alongside the need to have a better understanding of the cellular mechanisms to enable targeted treatments and improved safety is the need to measure inflammation more specifically. Only hsCRP is clinically used to quantify inflammation and this is non‐specific to CAD. Other inflammatory biomarkers such as IL‐6 and myeloperoxidase are associated with secondary cardiovascular events but are also non‐specific to CAD and influenced by factors such as age and body mass index.23,24 Currently, the timing for measurement of these biomarkers, particularly after an acute event, and overall utility of measuring these remain unclear. Further research is required to understand appropriate test and timing to ensure best response to these newly developing therapies. Future research, including the ZEUS trial,1 which has identified higher risk patients (elevated hsCRP) and targeting a downstream marker of inflammation (IL‐6), will provide insights into the best target population for these drugs.
The studies highlighted in this perspective article detail the multiple lines of evidence which show that targeting the immune system can benefit cardiac outcomes in the setting of CAD secondary prevention. Yet the identification of the population with residual risk due to inflammation needs to be carefully considered. There is still uncertainty about who has a net benefit from these treatments, but probably we have enough knowledge now to know that it is not everyone.
Box 1 – Antigens such as oxidised low‐density lipoprotein stimulate an innate and adaptive immune response*
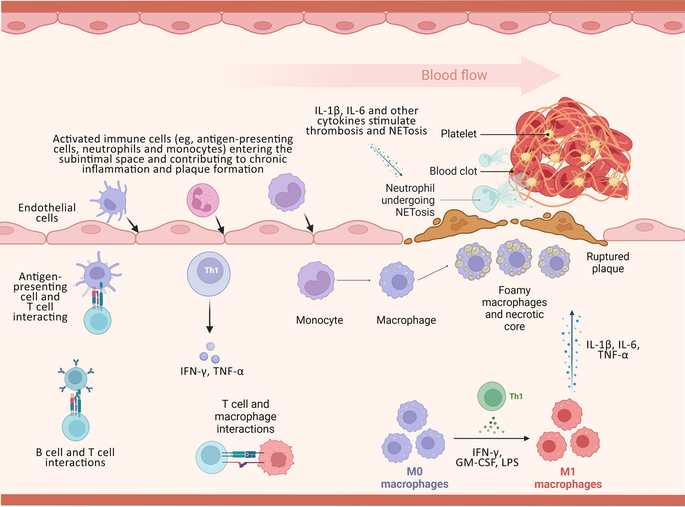
GM‐CSF = granulocyte‐macrophage colony‐stimulating factor; IFN = interferon; IL = interleukin; LPS = lipopolysaccharide; TNF = tumour necrosis factor. * This response stimulates T cells to release pro‐inflammatory cytokines and monocytes to develop into macrophages, with further release of inflammatory cytokines. This process predisposes to the development of atherosclerosis. When vulnerable plaque ruptures, it leads to thrombus formation. All aspects of this cascade are potential targets for therapeutic agents.1
Box 2 – Summary of previous and current clinical trials targeting the inflammatory pathway for secondary prevention of coronary artery disease
|
Clinical trial |
|||||||||||||||
|
Name (year) |
Drug |
Outcome measured |
Population |
Median follow‐up duration |
Notes/adverse events |
Methods |
Conclusion |
||||||||
|
|
|||||||||||||||
|
Low‐Dose Colchicine for Secondary Prevention of Cardiovascular Disease (LoDoCo; 2013)11 |
|
Acute coronary syndrome, OHCA, or non‐cardioembolic ischaemic stroke |
532 patients with stable coronary disease |
36 months |
|
Prospective, randomised, observer‐blinded, endpoint design |
|
||||||||
|
Canakinumab Anti‐inflammatory Thrombosis Outcomes Study (CANTOS; 2017)6 |
|
Non‐fatal MI, non‐fatal stroke, or CV death |
10 061 patients with prior MI and hsCRP ≥ 2 mg/L |
44.4 months |
|
Randomised, double‐blinded clinical trial |
|
||||||||
|
Colchicine Cardiovascular Outcomes Trial (COLCOT; 2019)12 |
|
Composite of death from CV causes, resuscitated cardiac arrest, MI, stroke, or urgent admission to hospital for angina leading to coronary revascularisation |
Patients recruited within 30 days of MI; 2366 patients were assigned to the colchicine group and 2379 to the placebo group |
22.7 months |
|
Randomised, double‐blind, placebo‐controlled trial |
|
||||||||
|
Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease 2 (LoDoCo2; 2020)13 |
|
Composite of CV death, spontaneous (non‐procedural) MI, ischaemic stroke, or ischaemia‐driven coronary revascularisation |
Chronic CAD;* 2762 patients were assigned to the colchicine group and 2760 to the placebo group |
28.6 months |
|
Randomised, controlled, double‐blind trial |
|
||||||||
|
Cardiovascular Inflammation Reduction Trial (CIRT; 2019)14 |
|
Composite of non‐fatal MI, non‐fatal stroke, or CV death. Before unblinding but near trial conclusion, hospital admission for unstable angina that led to urgent revascularisation was added to the primary endpoint |
4786 patients with stable CAD† |
27.6 months |
|
Randomised, double‐blind trial |
|
||||||||
|
Hydroxychloroquine for the Prevention of Cardiovascular Events in Myocardial Infarction Patients — a Safety Pilot Trial (OXI; 2021)15 |
|
Combination of acute MI, hospital admission due to recurrent unstable angina, hospital admission due to heart failure, and death within 1 year |
Pilot study, 125 patients enrolled within 96 hours of coronary angiography for NSTEMI or STEMI |
32 months |
|
Double‐blind, placebo‐controlled OXI trial |
|
||||||||
|
Phase 3 trials underway |
|
|
|
|
|
|
|
||||||||
|
CLEAR‐Synergy (ClinicalTrials.gov identifier NCT03048825) |
|
Inflammatory drug component endpoint: CV death, recurrent MI, or stroke in the colchicine comparison |
7000 patients with NSTEMI or STEMI |
24 months |
|
2 × 2 factorial randomised controlled trial |
|
||||||||
|
ZEUS1 |
|
CV death, non‐fatal MI and non‐fatal stroke |
6200 patients with chronic kidney disease and hsCRP ≥ 2 mg/L |
48 months |
|
Randomised clinical trial |
|
||||||||
|
|
|||||||||||||||
|
CAD = coronary artery disease; CI = confidence interval; CRP = C‐reactive protein; CV = cardiovascular; HR = hazard ratio; hsCRP = high‐sensitivity C‐reactive protein; IL = interleukin; LDL = low‐density lipoprotein; MI = myocardial infarction; NSTEMI = non‐ST‐elevation myocardial infarction; OHCA = out‐of‐hospital cardiac arrest; STEMI = ST‐elevation myocardial infarction. * Defined by CAD on invasive angiography or computed tomography coronary angiography or a calcium score of ≥ 400 Agatston units (1–99, mild disease; 100–399, moderate disease; > 400, severe disease) on calcium scan and stable for six months before enrolment. † Defined by previous MI or multivessel coronary disease in patients who additionally had either type 2 diabetes or metabolic syndrome. |
|||||||||||||||
Box 3 – Current therapeutic targets of the immune system for cardiovascular disease prevention1
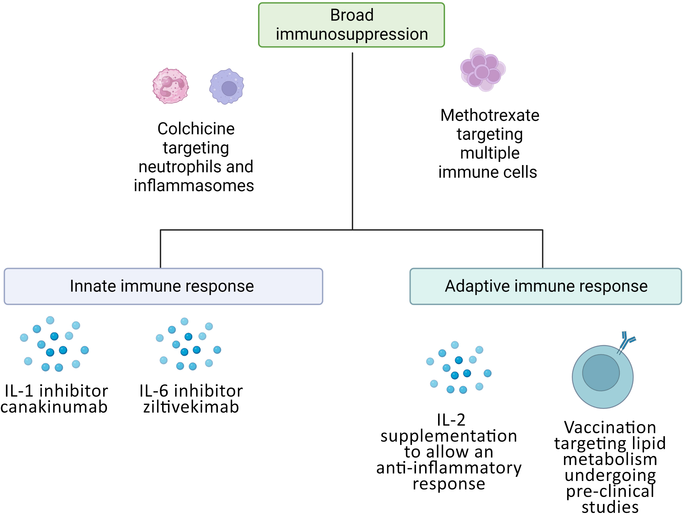
IL = interleukin.
Provenance: Commissioned; externally peer reviewed.
- 1. Engelen SE, Robinson AJB, Zurke YX, Monaco C, et al. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol 2022; 19: 522‐542.
- 2. Kazi SN, Von Huben A, Marschner S, et al. Trends in modifiable risk factors among first presentation ST elevation myocardial infarction patients in a large longitudinal registry. Heart Lung Circ 2023; 32: 480‐486.
- 3. Conrad N, Verbeke G, Molenberghs G, et al. Autoimmune diseases and cardiovascular risk: a population‐based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022; 400: 733‐743.
- 4. Wassif H, Saad M, Desai R, et al. Outcomes following acute coronary syndrome in patients with and without rheumatic immune‐mediated inflammatory diseases. J Am Heart Assoc 2022; 11: e026411.
- 5. Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973‐979.
- 6. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119‐1131.
- 7. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499‐511.
- 8. Hume RD, JJH Chong. The cardiac injury immune response as a target for regenerative and cellular therapies. Clin Ther 2020; 42: 1923‐1943.
- 9. Razeghian‐Jahromi I, Karimi Akhormeh A, Razmkhah M, Zibaeenezhad MJ. Immune system and atherosclerosis: hostile or friendly relationship. Int J Immunopathol Pharmacol 2022; 36: 3946320221092188.
- 10. Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol 2022; 22: 251‐265.
- 11. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low‐dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013; 61: 404‐410.
- 12. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med 2019; 381: 2497‐2505.
- 13. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383: 1838‐1847.
- 14. Ridker PM, Everett BM, Pradhan A, et al. Low‐dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380: 752‐762.
- 15. Ulander L, Tolppanen H, Hartman O, et al. Hydroxychloroquine reduces interleukin‐6 levels after myocardial infarction: The randomized, double‐blind, placebo‐controlled OXI pilot trial. Int J Cardiol 2021; 337: 21‐27.
- 16. Deftereos SG, Beerkens FJ, Shah B, et al. Colchicine in cardiovascular disease: in‐depth review. Circulation 2022; 145: 61‐78.
- 17. Ortega‐Paz L, Capodanno D, Angiolillo DJ. Canakinumab for secondary prevention of coronary artery disease. Future Cardiol 2021; 17: 427‐442.
- 18. Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 2020; 16: 145‐154.
- 19. Hays AG, et al. Randomized trial of anti‐inflammatory medications and coronary endothelial dysfunction in patients with stable coronary disease. Front Cardiovasc Med 2021; 8: 728654.
- 20. Fiolet ATL, Opstal TSJ, Mosterd A, et al. Efficacy and safety of low‐dose colchicine in patients with coronary disease: a systematic review and meta‐analysis of randomized trials. Eur Heart J 2021; 42: 2765‐2775.
- 21. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227‐3337.
- 22. Institut de Cardiologie de Montréal. Health Canada approval of low‐dose colchicine for cardiovascular disease based on the COLCOT study [media release]. 27 August 2021. https://www.icm‐mhi.org/en/pressroom/news/health‐canada‐approval‐low‐dose‐colchicine‐cardiovascular‐disease‐based‐colcot‐study (viewed Nov 2023).
- 23. McCarthy CP, McEvoy JW, Januzzi JL. Biomarkers in stable coronary artery disease. Am Heart J 2018; 196: 82‐96.
- 24. Gager GM, Biesinger B, Hofer F, et al. Interleukin‐6 level is a powerful predictor of long‐term cardiovascular mortality in patients with acute coronary syndrome. Vascul Pharmacol 2020; 135: 106806.






Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Clara Chow is the recipient of a National Health and Medical Research Council (NHMRC) Investigator Grant (APP1195326). James Chong was supported by an NHMRC Medical Research Future Fund Investigator Grant (APP1194139). The funding sources had no direct role in this study.
Clara Chow has been a speaker, panel member, or attended expert forums and received honoraria for a variety of pharmaceutical companies, including, in the past three years, Novartis, Amgen, Eli Lilly, Sandoz/Sanofi, and Novo Nordisk. Samia Kazi has been a speaker and panel member for Novo Nordisk.