The known: Immune checkpoint inhibitor therapy for people with advanced cutaneous squamous cell carcinoma (CSCC) is associated with impressive response rates and durable disease control in clinical trials.
The new: Among Australians with advanced CSCC treated with immune checkpoint inhibitors outside clinical trials during 2017–22, therapeutic benefit and toxicity rates were comparable with those reported by more selective registrational immunotherapy trials.
The implications: Immune checkpoint inhibitor therapy could be safe and effective for a broad range of people with advanced CSCC, including immunocompromised people and those aged 80 years or more.
The incidence of non‐melanoma skin cancers, including cutaneous squamous cell carcinoma (CSCC), in Australia and New Zealand is among the highest in the world, with age‐standardised incidence rates of 229.2 per 100 000 men and 66.7 per 100 000 women.1 Most people with CSCC present with local disease and can be cured; for those with advanced disease, however, median overall survival time is about fifteen months.2 Immune checkpoint inhibitors (ICIs) have revolutionised the management of advanced disease; 35–58% of people respond to treatment, and evidence for durable disease control, acceptable safety profiles, and improved quality of life have been reported.3,4,5,6,7
The typically higher age, greater frailty, and poorer performance status of people with advanced CSCC generally mean they do not meet the stringent criteria for inclusion in clinical trials. Similarly, people who are immunocompromised, which is associated with higher CSCC recurrence rates and poorer treatment outcomes, are generally excluded from trials.8,9 Interest in reviewing the outcomes of ICI therapy for people with advanced CSCC has therefore been great, but reports have been limited by small patient numbers.10,11,12,13,14,15
We therefore reviewed the outcomes of ICI therapy for people with advanced CSCC treated outside clinical trials and compared them with published clinical trial findings. Our aim was to provide an Australian perspective on treatment outcomes for a range of people who are often ineligible for clinical trials, and for whom treatment data are consequently not available.
Methods
We undertook a retrospective observational study, analysing data from fifteen Australian institutions for people with locally advanced (not amenable to curative radiotherapy or surgery, after discussion at a multidisciplinary meeting) or metastatic (nodal or distant) CSCC who received cemiplimab through a compassionate access program (Sanofi) under the Therapeutic Goods Administration Special Access Scheme, and a small number of people who personally covered the cost of pembrolizumab prior to the start of the access scheme. We included the fifteen institutions with the highest number of people with advanced CSCC treated in the access program. Adults (18 years or older) were eligible for cemiplimab under the access scheme if their hepatic function was adequate and they were ineligible for open cemiplimab trials. An Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 was generally required, but people with poorer ECOG status could be included on a case‐by‐case basis if deemed suitable by the treating physician. After recruitment for the phase II multi‐cohort registration study of cemiplimab for the treatment of advanced CSCC (https://clinicaltrials.gov/study/NCT02760498) closed on 15 February 2021, people who would have been eligible for this study could only receive cemiplimab via the access program and were therefore also included in our analysis.
Investigators at each participating site reviewed patient records for eligible cases and completed a standardised case report collection form (REDCap). The first included patient commenced treatment on 5 May 2017, the final one on 23 May 2022. People were treated with ICIs until death (any cause), unacceptable toxicity became evident, the disease progressed, or until a decision to end treatment by the patient or treating physician, for a maximum of two years. The reporting of our observational study conforms with STROBE guidelines.16
Outcomes
The primary endpoint was the investigator‐assessed best overall response rate (ORR) — that is, the proportion of treated patients with complete or partial responses to therapy, based on the overall number of response assessments — according to the hierarchy: the Response Evaluation Criteria in Solid Tumors (RECIST 1.1),17 the modified World Health Organization clinical response criteria,18 and the Positron Emission Tomography Response Criteria (PERCIST 1.0), based on [18F]‐fluorodeoxyglucose positron emission tomography (FDG‐PET)19 (Supporting Information, tables 1–3). If not assessed according to any of these criteria, a missing response assessment was recorded for the patient.
Secondary endpoints were overall survival, progression‐free survival, and toxicity.20 Tumour assessment intervals conformed with the usual standard of care. Overall survival was measured from commencement of ICI therapy to death from any cause, or censored at the final follow‐up assessment. Progression‐free survival was measured from commencement of ICI therapy to first documented evidence of disease progression or death from any cause. Data for people alive without evidence of progression were censored at the final follow‐up. Immunocompromised status was defined as the result of disease (ie, haematological malignancy), medication (systemic immunosuppressive treatment or prednisolone treatment exceeding 10 mg per day or equivalent for more than one month), or both.
Statistical analysis
Baseline and treatment data are summarised as descriptive statistics. The influence of selected clinico‐pathological prognostic features identified in previous reports10,11,12,13,14,15 was assessed in a Cox proportional hazards model (age, gender, head and neck primary site, ECOG performance status, immunocompromised status, metastatic disease and prior nodal radiotherapy). Survival, using log‐log transformation (log‐hazard), is depicted in Kaplan–Meier curves with 95% confidence intervals (CIs). To test whether relationships of overall survival and progression‐free survival with age were non‐linear, penalised spline modelling was used in exploratory analyses. Given concerns about prescribing systemic therapies to older people because of potential toxicity, we undertook an exploratory analysis of the influence of ECOG performance and immunocompromised status on survival by age group (under 80 years, 80 years or older). The statistical significance of differences in age, immunocompromised status, and autoimmune disease for people who did or did not report immune‐related adverse events of grade 2 or higher was assessed in independent sample t and χ2 tests. All statistical analyses were performed in R 4.2.1 (R Foundation for Statistical Computing).
Ethics approval
The study was reviewed and approved by the human research ethics committee of the Peter MacCallum Cancer Centre, Melbourne (HREC/76580/PMCC), which waived the requirement for individual consent to data access. This approval was accepted by the human research ethics committees of each of the participating institutions under the National Mutual Acceptance scheme.
Results
A total of 286 people with locally advanced or metastatic CSCC had received ICI therapy during May 2017 – May 2022, all of whom met our eligibility criteria; 270 received cemiplimab, 16 received pembrolizumab. Sixty‐two of those who received cemiplimab would have been eligible for the phase II registrational cemiplimab trial. The median follow‐up time was 12.2 months (interquartile range [IQR], 5.5–20.5 months). The median age of patients was 75.2 years (range, 39.3–97.5 years), 232 were men (81%), and 59 of 277 (21%) had ECOG performance scores of 2 or 3 (Box 1).
The primary site of cancer was the head and neck for 231 people (81%), and 98 patients (34%) had metastatic disease. Eighty‐eight people (31%) were immunocompromised. Fourteen (5%) had received renal transplants and two (1%) haematological transplants; 63 people (22%) had concurrent haematological malignancies, and 27 had autoimmune disease (9%), including rheumatoid arthritis (eight), psoriasis (five), and inflammatory bowel disease (four) (Box 1).
Response assessments
According to our hierarchical response assessment, the ORR was 60% (166 of 278 evaluable patients): complete responses were recorded for 74 (27%) and partial responses for 92 patients (33%); progressive disease was the best response for 55 people (20%) (Box 2). Thirteen patients experienced pseudo‐progression. Fourteen patients died before their first response assessment and were classified as having progressive disease.
For immunocompromised patients, the ORR was 51% (43 of 85 evaluable patients); for patients who were immunocompetent it was 64% (122 of 192 evaluable patients). A complete response was recorded for 19 (22%) and a partial response for 24 immunocompromised patients (28%) (Box 2).
Complete responses were recorded for one of twelve patients with active renal allografts and partial responses for three; stable disease was the best response for three people. Disease progressed in three people before their first imaging assessments, and for two others the best response was progressive disease. For people with haematological malignancy the ORR was 56% (35 of 62 evaluable patients) for those with chronic lymphocytic leukaemia it was 50% (12 of 24 evaluable patients).
Twenty‐two of the 55 people in whom disease progressed while receiving ICI therapy received additional systemic therapy, including sixteen who underwent chemotherapy.
Survival
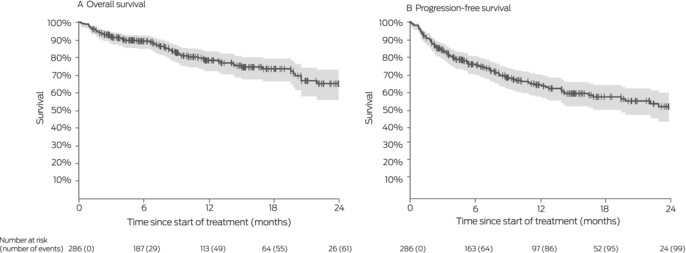
Twelve‐month overall survival was 78% (95% CI, 72–83%); progression‐free survival was 65% (95% CI, 58–70%) (Box 3). In multivariable analyses, poorer ECOG performance status was associated with poorer overall (per unit: adjusted hazard ratio [aHR], 3.0; 95% CI, 2.0–4.3) and progression‐free survival (aHR, 2.4; 95% CI, 1.8–3.3), as was being immunocompromised (overall: aHR, 1.8; 95% CI, 1.1–3.0; progression‐free: aHR, 1.8; 95% CI, 1.2–2.7). Progression‐free survival (but not overall survival) was better when the primary site of disease was the head or neck than for other regions (aHR, 0.6; 95% CI, 0.3–0.9). Age, gender, prior nodal irradiation, and disease status (locally advanced v metastatic) did not influence overall or progression‐free survival (Box 4).
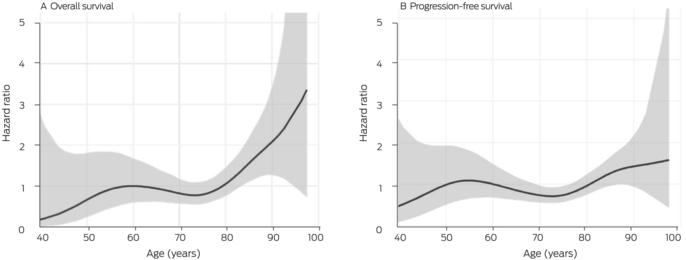
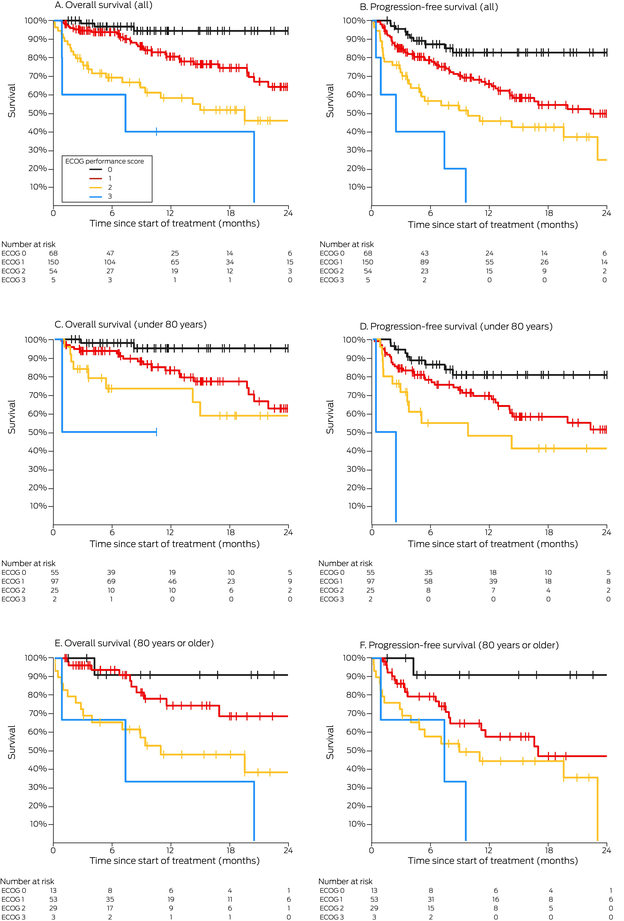
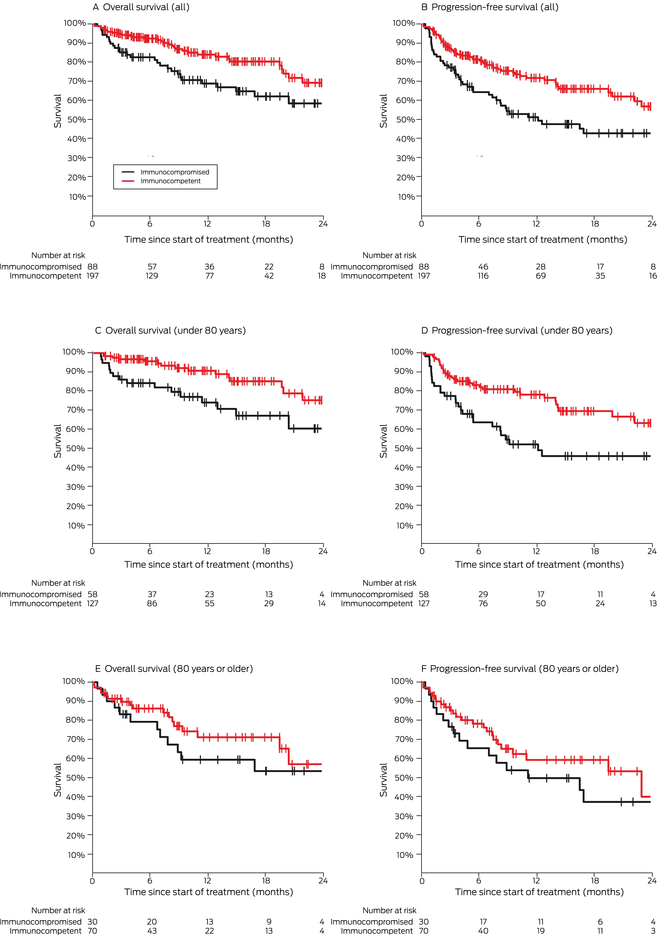
Our spline analysis indicated that the decline in overall and progression‐free survival was most marked from the age of 80 years (Box 5). The influence of ECOG performance status on survival was similar for people under 80 years of age and for those aged 80 years or more. Survival outcomes were best for people with ECOG performance scores of 0, and also clinically meaningful for those with ECOG scores of 1 or 2; survival was poor for the few patients with ECOG scores of 3 (Box 6). Kaplan–Meier analysis indicated that both overall and progression‐free survival were poorer for immunocompromised than immunocompetent patients in both age categories (Box 7).
Toxicity
Immune‐related adverse events were reported by 86 treated patients (30%), including 55 (19%) who reported events of grade 2 or higher; there were no treatment‐related deaths (Box 8). Thirty‐one people (11%) ended treatment because of toxicity. The mean and median ages of people who reported grade 2 or higher events were similar, as were the proportions of immunocompromised and immunocompetent patients who reported them; a larger proportion of people with autoimmune disease (12 of 27, 44%) than of those without autoimmune disease (43 of 259, 17%) experienced grade 2 or higher immune‐related adverse events (P = 0.001) (Box 9).
Fourteen patients had previously received renal transplants, twelve of whom had active grafts at the start of ICI therapy. Allograft rejection and subsequent graft loss was experienced by two patients during ICI treatment; both continued to receive ICI while on dialysis.
Discussion
Many people with advanced CSCC do not meet the stringent inclusion criteria of clinical trials. In our retrospective analysis of outcomes for Australians with advanced CSCC who received ICI treatment during 2017–22, including a large number who would have been ineligible for clinical trials, we found that its effectiveness and toxicity were consistent with outcomes reported by registration studies.3,4,5,6,7 Our analysis included the largest reported number of people with advanced CSCC treated with ICIs outside a clinical trial; further, the number of immunocompromised patients (88) was larger than in the key retrospective series reported in Italy (twelve)13 and France (59).21 As in other reports,3,6,13,21 most people in our series were men and over 70 years of age, but, in contrast to these reports, 93% had not yet received systemic therapy for advanced CSCC, better reflecting current clinical practice in which ICIs are used as first‐line therapy.
Our findings regarding the effectiveness of ICI therapy are consistent with those of most smaller retrospective series, which have reported ORRs of 50–60%10,13,21 (exception: 31.5% reported by a small United States study15). In our analysis, we estimated that 12‐month overall survival was 78% and progression‐free survival 65%. Direct comparisons with clinical trial outcomes are not possible, but in the phase II cemiplimab study 12‐month overall survival was 81% (95% CI, 68–89%) and progression‐free survival 53% (95% CI, 37–66%)3 and in the phase II pembrolizumab trial, 12‐month overall survival was 60% and progression‐free survival 32%.6 The RECIST 1.1 response assessment criteria used in both trials have notable limitations; they do not take into consideration pseudo‐progression (increased tumour size consistent with RECIST 1.1 progression because of inflammatory cell infiltration, but followed by tumour response to therapy), which affected thirteen people in our series. Further, FDG‐PET may be a better measure of depth of response than the RECIST 1.1 criteria.22
Disease status (locally advanced or metastatic disease) has not been identified as a key prognostic factor in registrational immunotherapy clinical trials of CSCC therapy, and its influence was not statistically significant in our multivariable survival analyses. However, poorer ECOG performance status was associated with poorer survival. In a French series, ECOG performance scores of 2 or more were associated with poorer survival, albeit only during the first six months of ICI therapy.21 While survival in our series for people with ECOG scores of 0 was remarkable, outcomes for many of those with ECOG scores of 1 or 2 were also more impressive than expected with chemotherapy or cetuximab.2,23 In the cemiplimab phase II trial, 12‐month progression‐free survival, as assessed by an independent central review, was 53%;3 survival outcomes for people in our series with ECOG performance scores of 2, although not as good as for those with scores of 0 or 1, were therefore still clinically significant. Further, the influence of ECOG status was statistically significant in our study among both people under 80 years and those aged 80 years or more. Clinicians can be hesitant about prescribing immunotherapy for people aged 80 years or older because the risk‐to‐benefit ratio is often presumed to be unfavourable, but our findings suggest that ECOG performance status is a predictor of outcomes, independent of age.
We found that survival outcomes were poorer for immunocompromised than for immunocompetent patients, in contrast to previous reports that included smaller numbers of immunocompromised people.13,21 Nevertheless, clinical benefit may still be derived from this otherwise highly effective therapy, if not to the same level as for immunocompetent people. We also found that toxicity was not higher for these patients, which suggests that ICI therapy can be safely used in this group. That the toxicity rate for our series was, in fact, similar to values reported by clinical trials3,6 is striking, given that we included people with other medical conditions and large numbers of people who were immunocompromised or with poorer ECOG performance status. This is an important finding, as clinicians can be hesitant about prescribing ICIs for people with autoimmune disease, immunosuppression, other medical conditions, or poorer ECOG performance status because of concerns about poorer response or greater toxicity.
Although treatment with corticosteroids or non‐selective immunosuppressants can reduce the response to ICIs,24,25,26 small case series suggest that selective immunosuppressants can be safely and effectively administered with ICIs to people with autoimmune disease.27,28,29 Our series included 27 people with autoimmune disease (9%), a group excluded from immunotherapy trials and some access programs;21 this number was larger than in similar studies.13 In contrast to these smaller studies, we found that the proportion of people who experienced immune‐related adverse events of grade 2 or higher was larger for those with autoimmune disease than for other patients. Most of these events, however, could be successfully managed, and there were no treatment‐related deaths.
Solid organ transplant recipients have been excluded from immunotherapy trials because of the high morbidity and mortality associated with allograft rejection. The magnitude of the rejection rate and the choice of immunosuppression for people receiving ICIs are, however, topics of debate. A recent Australian study found that maintaining baseline immunosuppression prior to treating people with renal transplants with ICIs may reduce the risk of rejection without altering the effectiveness of ICI treatment.30 A clinical trial investigating this question in renal transplant recipients with advanced CSCC is currently underway in the United States.31 Our series included twelve people with active renal allografts. We did not have information about their immunosuppression regimens, but only two experienced allograft rejection during ICI treatment, a smaller proportion than reported by other studies.30
Limitations
This study was limited by its retrospective nature; the reporting of comorbid conditions and adverse events, particularly low grade events, may have been incomplete. There was also no central assessment of disease response based on imaging, and no uniform choice of imaging modality or disease assessment interval. The sensitivity of FDG‐PET for assessing disease may have facilitated both more complete response assessments and better detection of progression, but it was only available for some patients. Follow‐up was relatively brief; longer follow‐up would better define the durability of responses and survival outcomes.
Conclusion
We analysed outcomes for a large group of Australians with advanced CSCC treated with ICIs outside clinical trials. Within the limitations of a retrospective analysis, our study adds to the evidence that appropriately selected people previously considered unfit for cytotoxic systemic therapy, including older people and those with poorer ECOG performance status or who are immunocompromised, may be safely and effectively treated with ICIs. ICI therapy could be beneficial for a broader range of patients with advanced CSCC than those eligible for participation in clinical trials.
Box 1 – Characteristics of 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022
|
Characteristic |
Value |
||||||||||||||
|
|
|||||||||||||||
|
Number of treated people |
286 |
||||||||||||||
|
Age (years) |
|
||||||||||||||
|
Mean (standard deviation) |
74.1 (11.2) |
||||||||||||||
|
Median (interquartile range) |
75.2 (66.8–82.8) |
||||||||||||||
|
Gender |
|
||||||||||||||
|
Men |
232 (81%) |
||||||||||||||
|
Women |
54 (19%) |
||||||||||||||
|
ECOG performance status |
|
||||||||||||||
|
0 |
68 (24%) |
||||||||||||||
|
1 |
150 (54%) |
||||||||||||||
|
2 |
54 (20%) |
||||||||||||||
|
3 |
5 (2%) |
||||||||||||||
|
Missing data |
9 |
||||||||||||||
|
Site of primary disease |
|
||||||||||||||
|
Head and neck region |
231 (81%) |
||||||||||||||
|
Trunk |
16 (6%) |
||||||||||||||
|
Limbs (upper and lower) |
28 (10%) |
||||||||||||||
|
Unknown primary, but presumed CSCC* |
11 (4%) |
||||||||||||||
|
Disease status at start of immunotherapy |
|
||||||||||||||
|
Distant metastatic disease |
98 (34%) |
||||||||||||||
|
Locally advanced |
188 (66%) |
||||||||||||||
|
Immunocompromised |
|
||||||||||||||
|
No |
197 (69%) |
||||||||||||||
|
Yes |
88 (31%) |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Haematological malignancy |
63 (22%) |
||||||||||||||
|
Chronic lymphocytic leukaemia |
24 [38%] |
||||||||||||||
|
Chronic lymphocytic leukaemia and other type |
1 [2%] |
||||||||||||||
|
Chronic myeloid leukaemia |
1 [2%] |
||||||||||||||
|
Follicular lymphoma |
9 [14%] |
||||||||||||||
|
Multiple myeloma |
3 [5%] |
||||||||||||||
|
Waldenström macroglobulinaemia |
4 [6%] |
||||||||||||||
|
Other |
21 [33%] |
||||||||||||||
|
Autoimmune disease |
27 (9%) |
||||||||||||||
|
Eczema |
2 [7%] |
||||||||||||||
|
Immune‐related haemolytic anaemia |
1 [4%] |
||||||||||||||
|
Inflammatory bowel disease |
4 [15%] |
||||||||||||||
|
Immune thrombocytopenic purpura and polymyalgia rheumatica |
1 [4%] |
||||||||||||||
|
Mastocytosis |
1 [4%] |
||||||||||||||
|
Pneumonitis |
1 [4%] |
||||||||||||||
|
Psoriasis |
5 [18%] |
||||||||||||||
|
Rheumatoid arthritis |
8 [30%] |
||||||||||||||
|
Sarcoidosis |
1 [4%] |
||||||||||||||
|
Systemic lupus erythematosis |
1 [4%] |
||||||||||||||
|
Vasculitis |
2 [7%] |
||||||||||||||
|
Previous systemic therapy |
|
||||||||||||||
|
None |
266 (93%) |
||||||||||||||
|
One line |
18 (6%) |
||||||||||||||
|
Two lines |
2 (< 1%) |
||||||||||||||
|
Previous radiotherapy for primary disease |
176 (62%) |
||||||||||||||
|
Previous surgery for primary disease |
223 (78%) |
||||||||||||||
|
Immune checkpoint inhibitor drug |
|
||||||||||||||
|
Cemiplimab |
270 (94%) |
||||||||||||||
|
Pembrolizumab |
16 (6%) |
||||||||||||||
|
Main reasons for compassionate access immunotherapy† |
|
||||||||||||||
|
Previous solid organ transplantation |
14 (5%) |
||||||||||||||
|
Haematological transplantation |
2 (1%) |
||||||||||||||
|
Haematological malignancy |
63 (22%) |
||||||||||||||
|
Autoimmune disease |
27 (9%) |
||||||||||||||
|
ECOG score > 1 or frail |
59 (21%) |
||||||||||||||
|
Chronic infection |
4 (1%) |
||||||||||||||
|
Concurrent other malignancy |
21 (7%) |
||||||||||||||
|
Recent other malignancy |
15 (5%) |
||||||||||||||
|
Previous immunotherapy |
6 (2%) |
||||||||||||||
|
Dialysis |
3 (1%) |
||||||||||||||
|
Primary CSCC lesion originated in red lip |
1 (< 1%) |
||||||||||||||
|
Primary CSCC lesion originated in conjunctiva |
1 (< 1%) |
||||||||||||||
|
Chronic renal impairment |
11 (4%) |
||||||||||||||
|
No RECIST 1.1 measurable disease |
27 (9%) |
||||||||||||||
|
Histology (ie, spindle cell component) |
7 (2%) |
||||||||||||||
|
Other |
39 (14%) |
||||||||||||||
|
Comorbid conditions |
|
||||||||||||||
|
0 |
121 (42%) |
||||||||||||||
|
1 |
85 (30%) |
||||||||||||||
|
2 |
56 (20%) |
||||||||||||||
|
3 |
17 (6%) |
||||||||||||||
|
4 |
7 (2%) |
||||||||||||||
|
Major comorbid condition |
|
||||||||||||||
|
Chronic kidney disease |
46 (16%) |
||||||||||||||
|
Liver disease |
1 (< 1%) |
||||||||||||||
|
Prior stroke or cerebrovascular accident |
14 (5%) |
||||||||||||||
|
Diabetes or metabolic syndrome |
52 (18%) |
||||||||||||||
|
Chronic obstructive pulmonary disease |
28 (10%) |
||||||||||||||
|
Dementia |
7 (2%) |
||||||||||||||
|
Other |
33 (12%) |
||||||||||||||
|
|
|||||||||||||||
|
ECOG = Eastern Cooperative Oncology Group. * Based on clinical history, pattern of disease, discussion in multidisciplinary meeting. † Multiple reasons possible. |
|||||||||||||||
Box 2 – Therapeutic response of 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022
|
|
Immunocompetent patients |
Immunocompromised patients |
All patients |
||||||||||||
|
Characteristic |
Number |
Proportion (95% CI) |
Number |
Proportion (95% CI) |
Number |
Proportion (95% CI) |
|||||||||
|
|
|||||||||||||||
|
Number of treated patients |
197 |
|
88 |
|
286* |
|
|||||||||
|
Best response† |
|
|
|
|
|
|
|||||||||
|
Complete response |
55 |
29% (22–36%) |
19 |
22% (14–33%) |
74 |
27% (22–32%) |
|||||||||
|
Partial response |
67 |
35% (28–42%) |
24 |
28% (19–39%) |
92 |
33% (27–39%) |
|||||||||
|
Stable disease |
37 |
19% (14–26%) |
20 |
24% (15–34%) |
57 |
21% (16–26%) |
|||||||||
|
Progressive disease |
33 |
17% (12–23%) |
22 |
26% (17–37%) |
55 |
20% (15–25%) |
|||||||||
|
Missing data |
5 |
|
3 |
|
8 |
|
|||||||||
|
Best RECIST 1.1 response |
|
|
|
|
|
|
|||||||||
|
Complete response |
26 |
23% (16–32%) |
8 |
18% (8–32%) |
34 |
22% (16–29%) |
|||||||||
|
Partial response |
47 |
42% (33–52%) |
17 |
38% (24–53%) |
65 |
41% (33–49%) |
|||||||||
|
Stable disease |
25 |
23% (15–31%) |
11 |
24% (13–40%) |
36 |
23% (17–30%) |
|||||||||
|
Progressive disease |
13 |
12% (6–19%) |
9 |
20% (10–35%) |
22 |
14% (9–21%) |
|||||||||
|
Missing data |
86 |
|
43 |
|
129 |
|
|||||||||
|
Best WHO clinical response |
|
|
|
|
|
|
|||||||||
|
Complete response |
15 |
27% (16–40%) |
3 |
15% (3–38%) |
18 |
24% (15–35%) |
|||||||||
|
Partial response |
22 |
39% (26–53%) |
8 |
40% (19–64%) |
30 |
39% (28–51%) |
|||||||||
|
Stable disease |
11 |
20% (10–32%) |
5 |
25% (9–49%) |
16 |
21% (13–32%) |
|||||||||
|
Progressive disease |
8 |
14% (6–26%) |
4 |
20% (6–44%) |
12 |
16% (8–26%) |
|||||||||
|
Missing data |
141 |
|
68 |
|
209 |
|
|||||||||
|
Best PERCIST 1.0 response |
|
|
|
|
|
|
|||||||||
|
Complete response |
30 |
45% (33–58%) |
13 |
45% (26–64%) |
43 |
45% (35–56%) |
|||||||||
|
Partial response |
19 |
29% (18–41%) |
7 |
24% (10–44%) |
26 |
27% (19–37%) |
|||||||||
|
Stable disease |
5 |
8% (3–17%) |
6 |
21% (8–40%) |
11 |
12% (6–20%) |
|||||||||
|
Progressive disease |
12 |
18% (10–30%) |
3 |
10% (2–27%) |
15 |
16% (9–25%) |
|||||||||
|
Missing data |
131 |
|
59 |
|
190 |
|
|||||||||
|
|
|||||||||||||||
|
CI = confidence interval; RECIST 1.1 = Response Evaluation Criteria in Solid Tumors;17 WHO = modified World Health Organization clinical response criteria;18 PERCIST 1.0 = Positron Emission Tomography Response Criteria.19 * Immunocompromised status for one patient could not be verified; they are consequently not included in the first two columns but are included in the third column because they were assessed (they achieved a partial response according to RECIST 1.1). † Hierarchy: RECIST 1.1 > modified World Health Organization clinical response criteria > PERCIST 1.0. |
|||||||||||||||
Box 3 – Survival for 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022: Kaplan–Meier analysis
Box 4 – Overall and progression‐free survival among 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022: Cox regression analyses
|
|
|||||||||||||||
|
|
|
Overall survival |
Progression‐free survival |
||||||||||||
|
Variable |
Patients |
Events |
Hazard ratio (95% CI) |
Adjusted hazard ratio* (95% CI) |
Events |
Hazard ratio (95% CI) |
Adjusted hazard ratio* (95% CI) |
||||||||
|
Age (per five years) |
286 |
66 |
1.2 (1.0–1.3) |
1.0 (0.9–1.2) |
103 |
1.1 (1.0–1.2) |
1.0 (0.9–1.1) |
||||||||
|
Gender |
|
|
|
|
|
|
|
||||||||
|
Men |
232 |
57 |
1 |
1 |
89 |
1 |
1 |
||||||||
|
Women |
54 |
9 |
0.7 (0.3–1.4) |
0.6 (0.3–1.3) |
14 |
0.7 (0.4–1.2) |
0.6 (0.4–1.1) |
||||||||
|
Primary site of disease |
|
|
|
|
|
|
|
||||||||
|
Head and neck |
231 |
50 |
0.7 (0.4–1.2) |
0.6 (0.3–1.1) |
77 |
0.6 (0.4–1.0) |
0.6 (0.3–0.9) |
||||||||
|
Other |
55 |
16 |
1 |
1 |
26 |
1 |
1 |
||||||||
|
ECOG performance status (per unit) |
277 |
65 |
2.7 (2.0–3.8) |
3.0 (2.0–4.3) |
101 |
2.2 (1.7–2.8) |
2.4 (1.8–3.3) |
||||||||
|
Immune status |
|
|
|
|
|
|
|
||||||||
|
Immunocompetent |
197 |
35 |
1 |
1 |
57 |
1 |
1 |
||||||||
|
Immunocompromised |
88 |
30 |
1.8 (1.1–3.0) |
1.8 (1.1–3.0) |
45 |
1.8 (1.2–2.7) |
1.8 (1.2–2.7) |
||||||||
|
Disease type |
|
|
|
|
|
|
|
||||||||
|
Locally advanced |
188 |
42 |
1 |
1 |
61 |
1 |
1 |
||||||||
|
Metastatic disease |
98 |
24 |
1.0 (0.6–1.6) |
0.9 (0.5–1.6) |
42 |
1.3 (0.8–1.9) |
1.1 (0.7–1.8) |
||||||||
|
Prior nodal radiotherapy |
|
|
|
|
|
|
|
||||||||
|
None |
191 |
41 |
1 |
1 |
63 |
1 |
1 |
||||||||
|
Prior nodal radiotherapy |
95 |
25 |
1.3 (0.8–2.1) |
1.3 (0.7–2.2) |
40 |
1.3 (0.9–2.0) |
1.2 (0.8–1.9) |
||||||||
|
|
|||||||||||||||
|
CI = confidence interval; ECOG = Eastern Cooperative Oncology Group. * Adjusted for all other variables in the table. |
|||||||||||||||
Box 5 – Relationship between survival and age for 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022: penalised spline analysis
Box 6 – Overall and progression‐free survival by ECOG performance status, overall and by age group (under 80 years, 80 years or older)
ECOG = Eastern Cooperative Oncology Group.
Box 7 – Overall and progression‐free survival by immunocompetence status, overall and by age group (under 80 years, 80 years or older)
Box 8 – Immune‐related adverse events reported by 286 people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) who received immune checkpoint inhibitor therapy, Australia, May 2017 – May 2022
|
Characteristic |
Number |
||||||||||||||
|
|
|||||||||||||||
|
Treated patients |
286 |
||||||||||||||
|
Immune‐related adverse events (any) |
86 (30%) |
||||||||||||||
|
Grade 2 or higher (any) |
55 (19%) |
||||||||||||||
|
Rheumatological immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
8 (3%) |
||||||||||||||
|
Grade 2 |
12 (4%) |
||||||||||||||
|
Grade 3 |
3 (1%) |
||||||||||||||
|
Endocrine immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
5 (2%) |
||||||||||||||
|
Grade 2 |
6 (2%) |
||||||||||||||
|
Grade 4 |
1 (< 1%) |
||||||||||||||
|
Skin immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
17 (6%) |
||||||||||||||
|
Grade 2 |
10 (4%) |
||||||||||||||
|
Grade 3 |
3 (1%) |
||||||||||||||
|
Pulmonary immune‐related adverse events |
|
||||||||||||||
|
Grade 2 |
2 (1%) |
||||||||||||||
|
Grade 3 |
5 (2%) |
||||||||||||||
|
Renal immune‐related adverse events |
|
||||||||||||||
|
Grade 3 |
1 (< 1%) |
||||||||||||||
|
Cardiac immune‐related adverse events |
|
||||||||||||||
|
Grade 3 |
1 (< 1%) |
||||||||||||||
|
Hepatic immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
1 (< 1%) |
||||||||||||||
|
Grade 2 |
1 (< 1%) |
||||||||||||||
|
Grade 3 |
1 (< 1%) |
||||||||||||||
|
Neurological immune‐related adverse events |
|
||||||||||||||
|
Grade 3 |
1 (< 1%) |
||||||||||||||
|
Colitis immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
1 (< 1%) |
||||||||||||||
|
Grade 2 |
1 (< 1%) |
||||||||||||||
|
Grade 3 |
3 (1%) |
||||||||||||||
|
Other immune‐related adverse events |
|
||||||||||||||
|
Grade 1 |
7 (2%) |
||||||||||||||
|
Grade 2 |
5 (2%) |
||||||||||||||
|
Grade 3 |
3 (1%) |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 9 – Characteristics of people with locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC), Australia, May 2017 – May 2022, by reporting of immune‐related adverse events (grade 2 or higher)
|
|
Grade 2 or higher immune‐related adverse events reported |
||||||||||||||
|
Characteristic |
No |
Yes |
|||||||||||||
|
|
|||||||||||||||
|
Treated patients |
231 |
55 |
|||||||||||||
|
Age (years) |
|
|
|||||||||||||
|
Mean (standard deviation) |
74.1 (11.1) |
73.9 (11.6) |
|||||||||||||
|
Median (interquartile range) |
75.2 (66.8–82.8) |
74.6 (67.4–82.7) |
|||||||||||||
|
Autoimmune disease |
|
|
|||||||||||||
|
No |
216 (83%) |
43 (17%) |
|||||||||||||
|
Yes |
15 (56%) |
12 (44%) |
|||||||||||||
|
Immunocompromised |
|
|
|||||||||||||
|
No |
162 (82%) |
35 (18%) |
|||||||||||||
|
Yes |
68 (77%) |
20 (23%) |
|||||||||||||
|
Missing data |
1 |
0 |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 11 May 2023, accepted 12 October 2023
- Luke S McLean1,2
- Annette M Lim1,2
- Mathias Bressel2,3
- Jenny Lee4,5
- Rahul Ladwa6,7
- Alexander D Guminski8
- Brett Hughes7,9
- Samantha Bowyer10
- Karen Briscoe11
- Samuel Harris12
- Craig Kukard13
- Rob Zielinski14,15
- Muhammad Alamgeer16,17
- Matteo Carlino18,19
- Jeremy Mo20
- John J Park21
- Muhammad A Khattak22,23
- Fiona Day24
- Danny Rischin1,2
- 1 Peter MacCallum Cancer Centre, Melbourne, VIC
- 2 The University of Melbourne, Melbourne, VIC
- 3 Centre for Biostatistics and Clinical Trials, Peter MacCallum Cancer Centre, Melbourne, VIC
- 4 Chris O'Brien Lifehouse, Sydney, NSW
- 5 Macquarie University, Sydney, NSW
- 6 Princess Alexandra Hospital, Brisbane, QLD
- 7 The University of Queensland, Brisbane, QLD
- 8 Northern Sydney Cancer Centre, Royal North Shore Hospital, Sydney, NSW
- 9 Royal Brisbane and Women's Hospital, Brisbane, QLD
- 10 Sir Charles Gairdner Hospital, Perth, WA
- 11 Mid North Coast Cancer Institute, Coffs Harbour, NSW
- 12 Bendigo Cancer Centre, Bendigo Health, Bendigo, VIC
- 13 Central Coast Cancer Centre, Gosford, NSW
- 14 Central West Cancer Care Centre, Orange, NSW
- 15 Western Sydney University, Penrith, NSW
- 16 Monash Health, Melbourne, VIC
- 17 Monash University, Melbourne, VIC
- 18 Melanoma Institute Australia, Westmead and Blacktown Hospitals, Sydney, NSW
- 19 The University of Sydney, Sydney, NSW
- 20 Westmead Hospital, Sydney, NSW
- 21 Nepean Cancer Care Centre, Penrith, NSW
- 22 Fiona Stanley Hospital, Perth, WA
- 23 Edith Cowan University, Perth, WA
- 24 Calvary Mater Newcastle, Newcastle, NSW
Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Data availability:
As this study involved multiple institutions, the study sponsor does not have authority to release the data generated by individual participating institutions. Any queries regarding the datasets generated and analysed for this study, however, can be directed to the corresponding author.
This study was funded by a National Health and Medical Research Council Investigator grant to Danny Rischin (APP1175929). Luke S McLean acknowledges the support of a National Health and Medical Research Council postgraduate scholarship. Cemiplimab was provided through a Sanofi Managed Access Program. Sanofi was not involved in the design, collection, analysis, interpretation or reporting of the data, but was given the opportunity to review the manuscript prior to submission. The decision to submit for publication was independently made by the authors.
Luke S McLean has received speaker fees and conference support from Sanofi, and conference registration and travel support from Merck Sharp & Dohme (MSD). Annette M Lim has served on the advisory board of MSD and Bristol‐Myers Squibb (uncompensated, apart from travel and accommodation expenses) and provided consultancy services to Eisai (uncompensated). Jenny Lee has received honoraria from MSD, Bristol Myers Squibb, and AstraZeneca, served on advisory boards of Sanofi and MSD, received conference support from Bristol Myers Squibb, Novartis, and MSD, and received research support from MSD. Rahul Ladwa has served on the advisory board and received speaker fees from Sanofi and MSD and received research funding from MSD (paid to his institution). Brett GM Hughes has served on advisory boards of MSD, Bristol Myers Squibb, Pfizer, Roche, Astra Zeneca, Sanofi, Takeda, Eisai, and Amgen, and received research funding from Amgen (paid to his institution). Rob Zielinski has received speaker fees from Sanofi. Matteo S Carlino has been a consultant advisor for Amgen, BMS, Eisai, Ideaya, MSD, Nektar, Novartis, Oncosec, Pierre‐Fabre, Qbiotics, Regeneron, Roche, Merck, and Sanofi, and received honoraria from BMS, MSD, and Novartis. Fiona Day has been a consultant for Amgen, received clinical trial support from BMS and AstraZeneca, and received travel support for speaker engagement from Merck. Danny Rischin has served on trial steering committees and advisory boards for MSD, GSK, Regeneron, and Sanofi (all uncompensated), received trial funding from MSD, Decibel Therapeutics, Bristol‐Myers Squibb, GSK, Roche, Regeneron, Kura Oncology, and ALX Oncology (paid to his institution).
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394‐424.
- 2. Cowey CL, Robert NJ, Davies K, et al. Treatment patterns and outcomes among patients with advanced cutaneous squamous cell carcinoma (CSCC) in a US community oncology setting [abstract: 2019 ASCO annual meeting I]. J Clin Oncol 2019;37 (15 Suppl): e21033.
- 3. Migden MR, Rischin D, Schmults CD, et al. PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018; 379: 341‐351.
- 4. Rischin D, Migden MR, Lim AM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed‐dosing, long‐term outcome of weight‐based dosing. J Immunoth Cancer 2020; 8: e000775.
- 5. Rischin D, Khushalani NI, Schmults CD, et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): longer follow‐up [abstract: 2020 ASCO annual meeting I]. J Clin Oncol 2020; 38 (15 Suppl): 10018.
- 6. Grob JJ, Gonzalez R, Basset‐Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single‐arm phase II trial (KEYNOTE‐629). J Clin Oncol 2020; 38: 2916‐2925.
- 7. Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open‐label, phase 2, single‐arm trial. Lancet Oncol 2020; 21: 294‐305.
- 8. Gonzalez JL, Reddy ND, Cunningham K, et al. Multiple cutaneous squamous cell carcinoma in immunosuppressed vs immunocompetent patients. JAMA Dermatol 2019; 155: 625‐627.
- 9. Manyam BV, Garsa AA, Chin RI, et al. A multi‐institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer 2017; 123: 2054‐2060.
- 10. Salzmann M, Leiter U, Loquai C, et al. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: real‐world data of a retrospective, multicenter study. Eur J Cancer 2020; 138: 125‐132.
- 11. Shalhout SZ, Park JC, Emerick KS, et al. Real‐world assessment of response to anti‐programmed cell death 1 therapy in advanced cutaneous squamous cell carcinoma. J Am Acad Dermatol 2021; 85: 1038‐1040.
- 12. Ríos‐Viñuela E, Álvarez P, Lavernia J, et al. Cemiplimab in advanced cutaneous squamous cell carcinoma: real‐world experience in a monographic oncology center. Actas Dermosifiliogr 2022; 113: 610‐615.
- 13. Baggi A, Quaglino P, Rubatto M, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer 2021; 157: 250‐258.
- 14. Hasmat S, Howle JR, Carlino MS, et al. Immunotherapy in advanced cutaneous squamous cell carcinoma: Sydney West Cancer Network experience. ANZ J Surg 2023; 93: 235‐241.
- 15. Hanna GJ, Ruiz ES, LeBoeuf NR, et al. Real‐world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI). Br J Cancer 2020; 123: 1535‐1542.
- 16. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344‐349.
- 17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228‐247.
- 18. World Health Organization. WHO handbook for reporting results of cancer treatment (WHO Offset Publication no 48). Geneva: WHO, 1979. https://apps.who.int/iris/bitstream/handle/10665/37200/WHO_OFFSET_48.pdf?sequence=1&isAllowed=y (viewed May 2023).
- 19. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (Suppl 1): 122S‐150S.
- 20. US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE), version 5.0. 27 Nov 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (viewed Dec 2023).
- 21. Hober C, Fredeau L, Pham‐Ledard A, et al. Cemiplimab for locally advanced and metastatic cutaneous squamous‐cell carcinomas: real‐life experience from the French CAREPI Study Group. Cancers (Basel) 2021; 13: 3547.
- 22. McLean LS, Cavanagh K, Hicks RJ, et al. FDG‐PET/CT imaging for evaluating durable responses to immune check point inhibitors in patients with advanced cutaneous squamous cell carcinoma. Cancer Imaging 2021; 21: 57.
- 23. Maubec E, Petrow P, Scheer‐Senyarich I, et al. Phase II study of cetuximab as first‐line single‐drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol 2011; 29: 3419‐3426.
- 24. Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune‐checkpoint inhibitors for cancer therapy: review of the literature and personalized risk‐based prevention strategy. Ann Oncol 2020; 31: 724‐744.
- 25. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol 2018; 36: 2872‐2878.
- 26. Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol 2018; 13: 1771‐1775.
- 27. Frohne CC, Llano EM, Perkovic A, et al. Complete response of metastatic melanoma in a patient with Crohn's disease simultaneously receiving anti‐α4β7 and anti‐PD1 antibodies. J Immunother Cancer 2019; 7: 1.
- 28. Uemura M, Trinh VA, Haymaker C, et al. Selective inhibition of autoimmune exacerbation while preserving the anti‐tumor clinical benefit using IL‐6 blockade in a patient with advanced melanoma and Crohn's disease: a case report. J Hematol Oncol 2016; 9: 81.
- 29. Kyi C, Carvajal RD, Wolchok JD, Postow MA. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer 2014; 2: 35.
- 30. Carroll RP, Boyer M, Gebski V, et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single‐arm, phase 1 study. Lancet Oncol 2022; 23: 1078‐1086.
- 31. Dharaneeswaran HJ, Giobbie‐Hurder A, Harran JJ, et al. Cemiplimab for kidney organ transplant recipients with advanced cutaneous squamous cell carcinoma: CONTRAC‐1 [abstract: 2023 ASCO annual meeting I]. J Clin Oncol 2023; 41 (16 Suppl): 9519.






Abstract
Objectives: To review the outcomes of immune checkpoint inhibitor (ICI) treatment of advanced cutaneous squamous cell carcinoma (CSCC) outside clinical trials.
Study design: Retrospective observational study; review of patient records in fifteen Australian institutions.
Setting, participants: All Australian adults with locally advanced or metastatic CSCC not amenable to curative surgery or radiotherapy treated with ICIs, 5 May 2017 – 23 May 2022, through a cemiplimab compassionate access scheme (Therapeutic Goods Administration Special Access Scheme) or who personally covered the cost of pembrolizumab prior to the start of the access scheme.
Main outcome measures: Best overall response rate (ORR) according to standardised assessment criteria using the hierarchy: Response Evaluation Criteria in Solid Tumors (RECIST 1.1), the modified World Health Organization clinical response criteria, and the Positron Emission Tomography Response Criteria (PERCIST 1.0); overall and progression‐free survival.
Results: A total of 286 people with advanced CSCC received ICI therapy during May 2017 – May 2022 (cemiplimab, 270; pembrolizumab, 16). Their median age was 75.2 years (range, 39.3–97.5 years) and 232 were men (81%); median follow‐up time was 12.2 months (interquartile range, 5.5–20.5 months). Eighty‐eight people (31%) were immunocompromised, 27 had autoimmune disease, and 59 of 277 (21%) had ECOG performance scores of 2 or 3. The ORR was 60% (166 of 278 evaluable patients): complete responses were recorded for 74 (27%) and partial responses for 92 patients (33%). Twelve‐month overall survival was 78% (95% confidence interval [CI], 72–83%); progression‐free survival was 65% (95% CI, 58–70%). Poorer ECOG performance status was associated with poorer overall survival (per unit: adjusted hazard ratio [aHR], 3.0; 95% CI, 2.0–4.3) and progression‐free survival (aHR, 2.4; 95% CI, 1.8–3.3), as was being immunocompromised (overall: aHR, 1.8; 95% CI, 1.1–3.0; progression‐free: aHR, 1.8; 95% CI, 1.2–2.7). Fifty‐five people (19%) reported immune‐related adverse events of grade 2 or higher; there were no treatment‐related deaths.
Conclusion: In our retrospective study, the effectiveness and toxicity of ICI therapy were similar to those determined in clinical trials. Our findings suggest that ICIs could be effective and well tolerated by people with advanced CSCC who are ineligible for clinical trials.