Clinical record
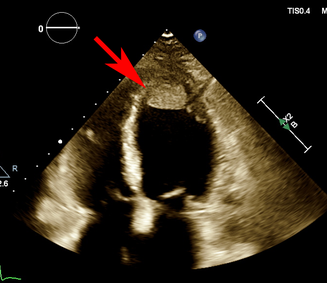
A 41‐year‐old G1P1 woman presented with two days of dyspnoea four weeks postpartum. She had an uncomplicated caesarean delivery at 39 weeks’ gestation in the setting of uterine fibroids, and she was breastfeeding. Her medical history was otherwise unremarkable. An electrocardiogram demonstrated sinus rhythm with anterolateral T‐wave inversion. A computed tomography (CT) pulmonary angiogram demonstrated no pulmonary embolus but pleural effusions and cardiomegaly. A transthoracic echocardiogram (TTE) demonstrated a left ventricular ejection fraction (LVEF) of 19% (normal range, ≥ 50%), left ventricular end‐diastolic diameter (LVEDD) of 5.3 cm (normal range, 3.5–5.6 cm) and a 1.8 × 2.3 cm mobile apical thrombus (Box 1). In the absence of risk factors, a diagnosis of peripartum cardiomyopathy (PPCM) was made. Frusemide, spironolactone, enalapril and metoprolol were initiated within 48 hours. The thrombus was managed with warfarin and bridging enoxaparin. Digoxin was commenced for inotropy. Bromocriptine was not started given the presence of thrombus. On day 3, the patient developed significant abdominal and flank pain. A CT scan of the abdomen and pelvis demonstrated extensive left renal and splenic infarctions. Repeat TTE demonstrated thrombus resolution and the LVEF was 25%. The surgical, nephrology, and interventional radiology teams were consulted for consideration of catheter‐directed thrombolysis. Consensus was for ongoing anticoagulation given the extensive infarction. The patient was registered with the Spleen Australia registry because of functional asplenia and was discharged on day 7. She remained well on review one week later. LVEF was 52% one month after discharge. Spironolactone was ceased at one month. LVEF was preserved at three months and renal function remained stable.
Discussion
PPCM remains a major cause of maternal mortality.1 It typically occurs towards the end of pregnancy, up to five months postpartum. Complications include heart failure, arrhythmias and venous thromboembolism. Hypercoagulability in pregnancy, cardiac dysfunction and dilation, stasis and endothelial dysfunction are thought to increase risk. Left ventricular thrombus has been reported in around 17% of patients2 with subsequent thromboembolic events in around 9% of women. Arterial thromboembolism such as limb ischaemia, stroke and myocardial infarction have been described with left ventricular thrombus.
Splenic and renal infarctions are rare. Without thrombosis, anticoagulation in PPCM is a class IIb recommendation for LVEF below 30% in the American Heart Association/American College of Cardiology/Heart Failure Society of America guidelines1 and for LVEF below 35% in the European Society of Cardiology guidelines.2 However, in patients with intracardiac thrombus, systemic embolism or atrial fibrillation, anticoagulation is strongly recommended.3 Other management options for renal infarction include catheter‐directed thrombolysis, systemic thrombolysis, and surgery. Catheter‐directed thrombolysis is considered in patients with complete occlusion of a major segmental renal artery with onset under six hours or in those with significant reduction in renal function or baseline renal failure.4
PPCM management postpartum includes current standard heart failure therapy (Box 2), except for sacubitril–valsartan and sodium–glucose cotransporter 2 (SGLT2) inhibitors, drug classes for which no safety data are yet available in breastfeeding.2 Enalapril and captopril are the preferred angiotensin‐converting enzyme inhibitors due to low levels in breast milk.1 Ventricular arrhythmias have been reported to occur in up to 18.7% of patients,5 but there are no established guidelines for implantable cardioverter‐defibrillator therapy. Bromocriptine, a prolactin inhibitor, has been found to improve survival in PPCM.1 A randomised multicentre trial in Germany found expedited left ventricular recovery and minimal adverse effects with both short and long term bromocriptine use.6 Bromocriptine inhibits lactation, which also enables the institution of early intensive standard heart failure therapy. However, bromocriptine has been associated with increased cerebral and cardiovascular complications and arterial thromboembolism, thus, concurrent anticoagulation is recommended.6 Only a few studies have reported safe use of bromocriptine in patients with existing thrombus.7
Prognosis in PPCM is typically better than in other forms of heart failure. Recovery typically occurs within three to six months,2 but it can be up to two years. Deterioration after initial recovery can occur, hence the importance of follow‐up. Increased LVEDD, LVEF below 35% at diagnosis, right ventricular dysfunction, African ancestry, older age, later diagnosis and elevated inflammatory markers confer poor prognosis.8 Late gadolinium enhancement on magnetic resonance imaging has also been shown to predict persistently reduced LVEF and higher risk of recurrence in future pregnancies.9 Guideline‐directed therapy is recommended in patients with ongoing cardiac dysfunction. However, in patients with recovered LVEF, the optimal duration of treatment remains unknown. Close monitoring of clinical status and LVEF is recommended, with stepwise cessation of medications.2 Stress echocardiogram and strain imaging have been propositioned as methods to detect subclinical left ventricular dysfunction but further research is required.
In women with persistent cardiac dysfunction, future pregnancy is discouraged as risk of PPCM recurrence is high and associated with high maternal and fetal mortality. In patients with recovered LVEF, the risk of recurrence is around 20%.2 Left ventricular recovery should be stable for three months after cessation of heart failure medications before conception.2 Our case report highlights the gravity and breadth of complications in PPCM and the importance of prompt institution of medical management.
Lessons from practice
- Peripartum cardiomyopathy (PPCM) is a major cause of mortality and morbidity in pregnancy.
- Renal and splenic infarctions are a rare complication of PPCM, with treatment options including anticoagulation and catheter‐induced thrombolysis.
- Bromocriptine can be considered in PPCM (class IIb recommendation) but safety in the presence of thrombus is unknown.
- Recovery in PPCM can be prolonged up to two years and relapse is common, hence the importance of ongoing surveillance. Recovery, in our patient, was seen within one month despite recognised poor prognostic factors such as left ventricular ejection fraction below 35%, right ventricular dysfunction and older maternal age, highlighting the importance of prompt institution of medical management.
Box 1 – Initial transthoracic echocardiogram demonstrating a 1.8 × 2.3 cm mobile apical thrombus (arrow)
Box 2 – Postpartum management of peripartum cardiomyopathy: recommended medications and considerations adapted from the American Heart Association/American College of Cardiology/Heart Failure Society of Amercia1 and the European Society of Cardiology4 guidelines
|
|
Class of recommendation,* level of evidence†,4 |
Considerations |
|||||||||||||
|
|
|||||||||||||||
|
Anticoagulation |
Class I, level A: recommended in patients with intracardiac thrombus, systemic embolism or atrial fibrillation |
Warfarin and heparin preferred |
|||||||||||||
|
ACE inhibitors |
Class I, level B: essential for all patients in standard or maximum tolerated dose |
Captopril or enalapril preferred |
|||||||||||||
|
ARB |
Class I, level B: recommended in patients who do not tolerate ACE inhibitors |
|
|||||||||||||
|
β‐blocker |
Class I, level B: essential for all patients in standard or maximum tolerated dose |
Selective β‐1‐blocker preferred; metoprolol preferred |
|||||||||||||
|
MRA |
Class I, level B: recommended in LVEF < 40% |
Potential fetal androgenic effects. Eplerenone preferred due to less hormonal side effects and less blood pressure reduction |
|||||||||||||
|
Loop diuretic |
Recommended in fluid overload |
|
|||||||||||||
|
Ivabradine |
Recommended in sinus rhythm with heart rate > 70 beats per minute at rest despite maximal tolerated β‐blocker |
|
|||||||||||||
|
Bromocriptine |
Class IIb, level B: may be considered to stop lactation and enhance LV function |
Must be accompanied by anticoagulation with heparin or at least in prophylactic dosages |
|||||||||||||
|
|
|||||||||||||||
|
ACE = angiotensin‐converting enzyme; ARB = angiotensin receptor blocker; LV = left ventricle; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist. * Classes of recommendations: class I: evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective; class II: conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure; class IIa: weight of evidence/opinion is in favour of usefulness/efficacy; class IIb: usefulness/efficacy is less well established by evidence/opinion; and class III: evidence or general agreement that the given treatment or procedure is not useful/effective and in some cases may be harmful. † Levels of evidence: level A: data derived from multiple randomised clinical trials or meta‐analyses; level B: data derived from a single randomised clinical trial or large non‐randomised studies; level C: consensus of opinion of the experts and/or small studies, retrospective studies, registries. Source: Table adapted from Bauersachs et al,4 with permission from Wiley and Sons (licence No. 5574170829359; 22 June 2023). |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145: e895‐e1032.
- 2. Davis MB, Arany Z, McNamara DM, et al. Peripartum cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75: 207‐221.
- 3. Bauersachs J, König T, van der Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology study group on peripartum cardiomyopathy. Eur J Heart Fail 2019; 21: 827‐843.
- 4. Mulayamkuzhiyil Saju J, Leslie SW. Renal Infarction. Treasure Island (FL): StatPearls Publishing, 2023.
- 5. Mallikethi‐Reddy S, Akintoye E, Trehan N, et al. Burden of arrhythmias in peripartum cardiomyopathy: analysis of 9841 hospitalizations. Int J Cardiol 2017; 235: 114‐117.
- 6. Hilfiker‐Kleiner D, Haghikia A, Berliner D, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 2017; 38: 2671‐2679.
- 7. Meyer GP, Labidi S, Podewski E, et al. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep 2010; 4: 80.
- 8. Iorgoveanu C, Zaghloul A, Ashwath M. Peripartum cardiomyopathy: a review. Heart Fail Rev 2021; 26: 1287‐1296.
- 9. Ricci F, De Innocentiis C, Verrengia E, et al. The role of multimodality cardiovascular imaging in peripartum cardiomyopathy. Front Cardiovasc Med 2020; 7: 4.





Patient consent
The patient gave written consent for publication.
No relevant disclosures.