Clinical record
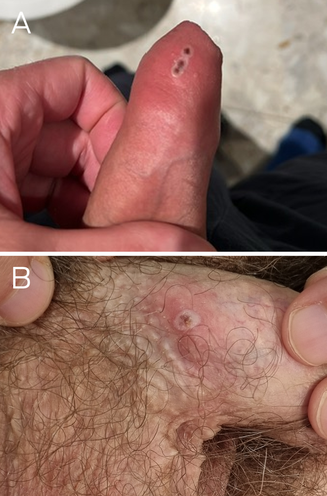
Patient 1, a 45‐year‐old man, presented with a ten‐day history of painless pustular penile lesions on 2 May 2023. He had a past history of appropriately treated syphilis but no other medical history. Patient 2, a 40‐year‐old man, presented with a three‐day history of a pustular penile lesion on 9 May 2023. He had no significant medical history. Neither patient reported systemic symptoms, fever or lymphadenopathy. Both reported sexual contact with men, were human immunodeficiency virus (HIV) negative, and were taking HIV pre‐exposure prophylaxis. Both men reported sexual contact nine days before symptom onset with an asymptomatic casual male partner from Europe, but it was unknown whether they shared the same contact. Examination of patient 1 revealed a cluster of small pustules on the distal penile shaft, without regional lymphadenopathy (Box, A). Examination of patient 2 showed a small, scabbed pustule on the proximal penile shaft with mild surrounding erythema and bilateral non‐tender inguinal lymphadenopathy (Box, B). The lesions were swabbed and tested for herpes simplex virus, Treponema pallidum and monkeypox virus (MPXV) by polymerase chain reaction (PCR). Other investigations included serology for HIV and T. pallidum, and screening for Chlamydia trachomatis and Neisseria gonorrhoeae on first pass urine, and oropharyngeal and anorectal swabs using PCR. Testing for MPXV, conducted using the Novaplex MPXV assay (Seegene Technologies) at New South Wales Health Pathology, was positive for both men. No concomitant sexually transmissible infections were detected. Tests for Orthopoxvirus antibodies, with an in‐house assay at the Institute of Clinical Pathology and Medical Research, on eight‐week‐old pre‐exposure serum stored from previous sexually transmissible infections screening (routine serum storage as per local laboratory procedure) from patient 1 and acute serum from patient 2, were negative for IgG and IgM antibodies against both MPXV and vaccinia virus targets.
Patient 1 had received two doses (0.1 mL each) of third generation smallpox vaccine (modified vaccinia virus Ankara–Bavarian Nordic [MVA‐BN], Jynneos) intradermally four weeks apart, with his last dose six months before the infection. Patient 2 had received the first dose subcutaneously (0.5 mL) and his second dose three months later intradermally (0.1 mL) — the last dose was received seven months before infection. Both men required conservative management only and were advised to avoid sexual contact until lesions had healed. Patient 2 developed a mild secondary bacterial infection of the penile lesion that was responsive to five days of oral cephalexin 500 mg four times a day. Seven contacts were identified in total, of whom four received post‐exposure prophylaxis with MVA‐BN vaccination. Vaccination status of the European sexual partner was unknown. As required, cases were notified to the relevant public health unit. All other sexual contacts, with the exception of the European sexual contact, were monitored and remain asymptomatic to date.
Discussion
Since May 2022, there has been a global outbreak of mpox (formerly monkeypox), predominantly in gay, bisexual and other men who have sex with men.1 In Australia, there have been a total of 154 cases to date, most of whom have acquired their infections overseas, mainly in Europe and North America.2 Before these two cases, the last reported case in Australia was in November 2022. Most cases only require supportive management, as antiviral treatment such as tecovirimat and vaccinia immune globulin is rarely indicated.1 Sexual contacts within three weeks of index case symptoms are managed according to vaccination status. Unvaccinated contacts within 14 days of exposure are eligible for mpox post‐exposure prophylaxis with the MVA‐BN vaccine; although vaccination within four days of exposure provides the greatest chance of preventing disease, later vaccination may nonetheless attenuate disease severity. Unvaccinated contacts outside of this time are advised to receive mpox pre‐exposure/primary vaccination. Vaccinated contacts are monitored for symptoms.
In Australia, vaccination against mpox with third generation smallpox vaccine was rolled out to high risk groups in August 2022. These groups include gay, bisexual and other men who have sex with men; sex workers; and sexual partners and other close contacts of people in these groups.1 Primary vaccination with MVA‐BN involves the administration of two doses at least 28 days apart either subcutaneously or intradermally. Intradermal vaccination, using 20% of the subcutaneous dose, has been shown to be non‐inferior to subcutaneous vaccination in terms of immunogenicity, and was used as a dose‐sparing strategy in response to constrained vaccine supplies.1,3,4 Serum antibody studies indicate that MVA‐BN induces a protective immunological response that peaks two weeks after the second dose. Studies in naïve vaccine recipients demonstrate a decline in IgG titres such that about 40% of individuals are seronegative six months after a two‐dose vaccination schedule.5 Data on the vaccine's clinical efficacy and durability against human MPXV infection are limited.1,4,6 A recent publication measured overall real‐world vaccine effectiveness at 85.9% (95% confidence interval [CI], 73.8–92.4%) after full vaccination, with adjusted vaccine efficacy of 88.9% (95% CI, 56.0–97.2%) for full vaccination by subcutaneous route, 80.3% (95% CI, 22.9–95.0%) for full vaccination by intradermal route, and 86.9% (95% CI, 69.1–94.5%) for heterologous vaccination.3 Another publication estimated lower overall vaccine effectiveness at 66.0% (95% CI, 47.4–78.1%) for full vaccination.4
One large clinical observation study of people diagnosed with mpox in the Netherlands, mainly unvaccinated gay, bisexual and other men who have sex with men, demonstrated systemic symptoms in 86% of cases, usually occurring before the onset of cutaneous disease.7 Most patients (63%) had lesions on at least two different body locations. Both of our cases had limited cutaneous disease and no spread outside the presumed inoculation site and without systemic symptoms. A similar clinical course was also observed in a cluster of 22 mpox cases in Chicago consisting of fully vaccinated individuals (median time since last vaccine, 8.4 months; interquartile range, 7.9–8.8 months) who also experienced mild self‐limiting illness.8 This observation may suggest that MVA‐BN is effective at preventing severe disease even if a breakthrough infection does occur. Perhaps the absence of circulating antibodies permitted symptomatic local infection to establish, but the anamnestic immune response may then have prevented systemic dissemination. Clinicians should be aware of the possibility of breakthrough modified MPXV infection in patients with epidemiological risk factors and a characteristic vesiculopustular rash, despite a history of vaccination. The durability of the vaccine efficacy remains unknown, including the need for a future vaccine booster should the epidemiological risk of mpox persist.
Lessons from practice
- Since May 2022, there has been a global outbreak of mpox, predominantly in gay, bisexual and other men who have sex with men.
- Limited data exist for clinical vaccine effectiveness, but it has been estimated at about 85% after a full vaccination course.
- Clinicians should be aware of the possibility of breakthrough infection, possibly of less severity and without typical constitutional symptoms, in patients with epidemiological risk factors and a characteristic vesiculopustular rash, irrespective of a history of previous vaccination.
- Individuals at high risk should be offered vaccination, if not yet already received. These groups include gay, bisexual and other men who have sex with men; sex workers; and sexual partners of these groups.
Provenance: Not commissioned; externally peer reviewed.
- 1. Australian Technical Advisory Group on Immunisation. Updated ATAGI clinical guidance on vaccination against monkeypox (mpox). Canberra: Commonwealth of Australia, 2022. https://www.health.gov.au/sites/default/files/2022‐12/atagi‐clinical‐guidance‐on‐vaccination‐against‐monkeypox.docx (viewed May 2023).
- 2. Department of Health and Aged Care. National Notifiable Disease Surveillance System. Canberra: Commonwealth of Australia, 2023. https://nindss.health.gov.au/pbi‐dashboard/ (viewed July 2023).
- 3. Dalton AF, Diallo AO, Chard AN, et al. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: a multijurisdictional case–control study — United States, August 19, 2022 – March 31, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 553‐558.
- 4. Deputy NP, Deckert J, Chard A, et al. Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N Engl J Med 2023; 388: 2434‐2443.
- 5. Priyamvada L, Carson WC, Ortega E, et al. Serological responses to the MVA‐based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine 2022; 40: 7321‐7327.
- 6. Xu M, Liu C, Du Z, et al. Real‐world effectiveness of mpox (monkeypox) vaccines: a systematic review. J Travel Med 2023; doi: https://doi.org/10.1093/jtm/taad048 [Epub ahead of print].
- 7. van Ewijk CE, Miura F, van Rijckevorsel G, et al. Mpox outbreak in the Netherlands, 2022: public health response, characteristics of the first 1,000 cases and protection of the first‐generation smallpox vaccine. Euro Surveill 2023; 28: 2200772.
- 8. Faherty EA, Holly T, Ogale YP et al. Emergence of an mpox cluster primarily affecting persons previously vaccinated against mpox — Chicago, Illinois, March 18 – June 12, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 696‐697.
- 1. Australian Technical Advisory Group on Immunisation. Updated ATAGI clinical guidance on vaccination against monkeypox (mpox). Canberra: Commonwealth of Australia, 2022. https://www.health.gov.au/sites/default/files/2022‐12/atagi‐clinical‐guidance‐on‐vaccination‐against‐monkeypox.docx (viewed May 2023).
- 2. Department of Health and Aged Care. National Notifiable Disease Surveillance System. Canberra: Commonwealth of Australia, 2023. https://nindss.health.gov.au/pbi‐dashboard/ (viewed July 2023).
- 3. Dalton AF, Diallo AO, Chard AN, et al. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: a multijurisdictional case–control study — United States, August 19, 2022 – March 31, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 553‐558.
- 4. Deputy NP, Deckert J, Chard A, et al. Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N Engl J Med 2023; 388: 2434‐2443.
- 5. Priyamvada L, Carson WC, Ortega E, et al. Serological responses to the MVA‐based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine 2022; 40: 7321‐7327.
- 6. Xu M, Liu C, Du Z, et al. Real‐world effectiveness of mpox (monkeypox) vaccines: a systematic review. J Travel Med 2023; doi: https://doi.org/10.1093/jtm/taad048 [Epub ahead of print].
- 7. van Ewijk CE, Miura F, van Rijckevorsel G, et al. Mpox outbreak in the Netherlands, 2022: public health response, characteristics of the first 1,000 cases and protection of the first‐generation smallpox vaccine. Euro Surveill 2023; 28: 2200772.
- 8. Faherty EA, Holly T, Ogale YP et al. Emergence of an mpox cluster primarily affecting persons previously vaccinated against mpox — Chicago, Illinois, March 18 – June 12, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 696‐697.






Patient consent
The patients gave written consent for publication.
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
Although the World Health Organization recommended changing the name of the disease caused by monkeypox virus to “mpox”, the name of the virus remains “monkeypox virus”. Thus, the term “monkeypox virus” is used when referring to an infection, whereas “mpox” is used when referring to the disease.
No relevant disclosures.