Glucocorticoids are widely used in medicine, beginning with the revolutionary use of cortisone in rheumatoid arthritis, shortly after its synthesis in 1950.1 Inactive cortisone is converted endogenously to active hydrocortisone. Subsequently, Arthrobacter microbiological dehydrogenation introduced a 1,2‐corticoid ring double bond, leading to prednisolone, which has four times the anti‐inflammatory potency as hydrocortisone but less salt‐retaining properties, a longer plasma disappearance half‐life, and tissue effect compatible with once daily dosing.2,3 Hence, prednisolone is the preferred anti‐inflammatory oral glucocorticoid. Glucocorticoids are perhaps the most versatile of all medical therapies, leading to disease control, often life saving, in at least 50 discrete diseases.4 Nevertheless, glucocorticoids risk severe adverse reactions, particularly if taken in high systemic doses for a prolonged period. Use of prednisolone in supraphysiological doses (> 5 mg per day) or steroid equivalents (Box 1) for at least 21 days was reported in 14% of inhabitants of western Sweden over a seven‐year period.5 Population prevalence of long term oral glucocorticoid use is estimated at about 1–3%.6,7 The glucocorticoids side effects comprise metabolic (central fat mass increase, hyperglycaemia, hypertension), catabolic (skin thinning, proximal muscle atrophy, bone loss) and neurocognitive and mood disturbances, together representing exogenous Cushing syndrome. Typically, systemic glucocorticoids are given at a dose sufficient to control disease, then withdrawn at a rate that could minimise adverse withdrawal reactions, risk of adrenal insufficiency and disease reactivation.
Glucocorticoid weaning has not been subject to rigorous clinical trial due to the many individual variables involved. These include variable metabolism of the administered glucocorticoids, the extent of glucocorticoid withdrawal symptoms and the activity of the disease that led to glucocorticoid use. Hence, glucocorticoid withdrawal schemes are generally based on expert opinion rather than empirical evidence.8,9 The safety of abrupt glucocorticoid cessation has not been studied.
The use of a morning plasma cortisol or short synacthen test (SST) to evaluate adrenal reserve to determine the weaning rate of glucocorticoid is widespread.10 Unfortunately, their value in predicting actual adrenal insufficiency or adrenal crisis events on glucocorticoid withdrawal has not been systematically studied, and borderline values need to be interpreted with caution. This article aims to provide guidance to clinicians for a safe and effective way to wean patients off long term glucocorticoids.
Glucocorticoid daily doses greater than those used for physiological replacement for more than three to four weeks often lead to long term suppression of the hypothalamic–pituitary–adrenal axis, which operates to provide life‐sustaining cortisol without excess.3
Adrenal suppression
Adrenal suppression results from prolonged negative feedback by glucocorticoids on cortisol secretion (Box 2). Adrenal suppression arises from glucocorticoids acting via glucocorticoid receptors in key brain structures, the paraventricular nucleus of the hypothalamus, the hippocampus, the amygdala and the prefrontal cortex and mineralocorticoid receptors in limbic structures.11,12 In addition, feedback occurs via glucocorticoid receptors in the pituitary and adrenal cortex.12 The paraventricular nucleus corticotropin‐releasing hormone (CRH) neurons are critical in hypothalamic–pituitary–adrenal axis regulation and they (or contiguous brain structures) primarily underlie the phenomenon of prolonged glucocorticoid suppression in humans and non‐human primates.13 Direct suppression of adrenocorticotropic hormone (ACTH)‐secreting pituitary corticotropes and adrenocortical atrophy, particularly at the cortisol secreting zona fasciculata also develop.14 The primacy of central sites of glucocorticoid suppression is suggested by a study of patients with Cushing syndrome after removal of their pituitary adenoma, where prolonged suppression of cortisol and ACTH (typically over six to 18 months) is seen, but an immediate post‐operative infusion of CRH normalises cortisol secretion.14
Glucocorticoid weaning may be met with symptoms of adrenal insufficiency (fatigue, nausea, anorexia, diffuse aches and pains) or adrenal crisis (adrenal insufficiency symptoms plus absolute or relative hypotension that responds rapidly, within one hour, to exogenous hydrocortisone, excluding other pathologies), and/or symptoms of hormone withdrawal syndrome.15 Patients with primary or secondary adrenal insufficiency have established disease of the adrenal cortex or the pituitary and/or hypothalamus.3 Hypothalamic‐based adrenal insufficiency may be referred to as tertiary adrenal insufficiency, but, from a clinical viewpoint, disease of the hypothalamic and/or pituitary unit is often considered together as both result from ACTH deficiency.3 Both primary and secondary adrenal insufficiency incur a risk of adrenal crisis — an acute emergency where cortisol production is insufficient to maintain homeostasis — considering that higher cortisol concentrations are needed in the face of states where tissue glucocorticoid resistance occurs, such as inflammation or trauma. Adrenal crisis risk in patients with primary and secondary adrenal insufficiency is substantial, at 8% and 6% per annum respectively.15 Glucocorticoid‐induced adrenal insufficiency is generally considered common but entails a substantially lower risk of adrenal crisis. An Australian epidemiological study of glucocorticoid‐induced adrenal insufficiency showed a low rate of adult hospital admissions for adrenal insufficiency and rare admissions for adrenal crises.16
Hormone withdrawal syndrome, where symptoms such as fatigue, nausea, body aches and mood disturbance develop, can be confused with absolute adrenal insufficiency, but may occur at supraphysiological glucocorticoid doses. These symptoms are seen with withdrawal of other hormones and opiates and are postulated to have a unifying central nervous system mechanism.17,18
Adrenal crisis risk may be minimised by the following advice:15- In case of illness, especially in the presence of fever, a “stress dose” of glucocorticoids can be delivered, where the aim is to mimic the usual adrenocortical response. This typically involves tripling the usual glucocorticoid dose, unless glucocorticoid dosing is already in the threefold physiological range or above.
- When oral hydrocortisone is not practical, such as in vomiting, use injectable hydrocortisone 100 mg (intramuscular or subcutaneous) via the Solu‐Cortef Act‐O‐Vial (Pfizer) device, which requires specific practical instruction for mixing, draw up and injection.
- Advise wearing of a MedicAlert bracelet, necklace or similar to indicate the need for parenteral hydrocortisone when the patient may be found unwell and unable to communicate.
Specific glucocorticoid dosing regimens for surgery, based on the extent of the surgical procedure, are available at the Addison's Disease Self‐Help Group website.19 Assiduous “sick day” education along these lines may reduce the risk of adrenal crisis, but residual risk remains and is unevenly distributed among individual patients for uncertain reasons.16,20
The extent and duration of adrenal suppression after exogenous glucocorticoid exposure varies, but generally only occurs after at least three weeks of supraphysiological glucocorticoid use.16 The duration may be as long as 12–18 months after glucocorticoid withdrawal, as revealed after Cushing syndrome,21 but most patients with glucocorticoid‐induced adrenal insufficiency are at risk for shorter intervals.16
No algorithm has been developed to clinically predict the risk of adrenal insufficiency after glucocorticoid withdrawal in the individual patient. A systematic review and meta‐analysis showed that glucocorticoid dose and duration, reflected also in glucocorticoid administrative route and underlying disease, predict the risk of biochemically diagnosed adrenal insufficiency.22 The highest risk is seen in patients taking oral glucocorticoids (48.7%) and intra‐articular forms (52%), with lower rates for inhaled (7.8%), topical (4.7%) and nasal (4.2%) glucocorticoids.22 There is a wide variability in glucocorticoid activity of a given oral dose in different individuals due to differences in absorption, metabolism, glucocorticoid receptor expression, and downstream gene activation.12,23,24 High intra‐individual variability in glucocorticoid sensitivity is apparent in estimates of daily cortisol production rates at 9.9 mg per day (standard deviation, 2.7 mg per day) and a threefold variation in 24‐hour urine‐free cortisol in healthy individuals.25
Several tests have been used to assess cortisol secretion and the extent of glucocorticoid suppression from exogenous glucocorticoids. These tests include morning plasma cortisol, taking advantage of the peak of cortisol circadian rhythm at 6–9 am, shortly after waking, where cortisol concentrations are sevenfold those at nadir (midnight) in the presence of a typical sleep–wake cycle, as the rhythm is entrained by light. Morning cortisol often obviates the need for an SST, but SST is more reproducible.26 The SST uses the ACTH 1‐24 (cosyntropin, synacthen) N‐terminal fragment of the full 39‐amino acid ACTH peptide to stimulate adrenal cortisol secretion. The test relies on the principle that adrenal suppression will eventually lead to adrenocortical atrophy (after about six weeks, as evidenced after pituitary surgery) and, hence, a subnormal response to administered synacthen will be observed. Alternative tests, such as insulin hypoglycaemia, metyrapone stimulation of ACTH and 11‐deoxycortisol, CRH stimulation and morning salivary cortisone or cortisol are not routinely used in this context due to safety, side effects or expense, as well as insufficient supporting interpretative data in this context.
Morning and SST‐stimulated cortisol estimates vary between assays, so assay‐specific control values should be used.27 A gap between the administered glucocorticoid and serum cortisol is essential for assessment of endogenous cortisol, which is 12 hours for hydrocortisone and 24 hours for prednisolone and dexamethasone.3 Cortisol circulates bound in high affinity to corticosteroid‐binding globulin (80%), low affinity to albumin (15%) and free in plasma (5%) at physiological cortisol concentrations.28 A greater proportion is free when cortisol exceeds the corticosteroid‐binding globulin concentration (~500 nmol/L).28 Conditions that may produce hypoproteinaemia, such as liver disease or nephrotic syndrome, may reduce bound cortisol. Conventional cortisol assays measure both bound and free cortisol and will be lowered in hypoproteinaemia, falsely implying low free cortisol that can diffuse through cell membranes.28
Glucocorticoid weaning
Many patients are treated with glucocorticoids until suppression or remission of the underlying inflammatory disease or the use of alternative disease‐suppressing agents. In clinical practice, advice regarding glucocorticoid weaning while minimising the risk of adrenal insufficiency and adrenal crisis is often sought. We propose the following, which has worked well in our tertiary hospital setting for over ten years. Weaning glucocorticoids require consideration of three risks:- reactivation of the disease that prompted the use of glucocorticoids;
- hormone withdrawal syndrome;
- adrenal insufficiency or adrenal crisis.
The rate of glucocorticoid weaning relates to these three risks and is quantitatively informed by measures of endogenous cortisol production. High disease activity is likely to prohibit glucocorticoid weaning, at least until there is a remission or alternative effective therapies are in place. Hormone withdrawal syndrome may develop with rapid glucocorticoid withdrawal above the physiological range. Adrenal insufficiency or adrenal crisis may develop with glucocorticoid withdrawal below the physiological range.
Practically, these three considerations and cortisol measurements (morning cortisol or, if needed, an SST) should guide the rate of glucocorticoid withdrawal. Patients should be advised to report adrenal insufficiency symptoms and resume the previous dose should these occur after a weaning dose reduction step. Prevention measures for adrenal crisis events should be in place until 12 months after cessation of glucocorticoids.
Morning cortisol and cortisol level after 250 μg intravenous or intramuscular SST (30‐ or 60‐minute post‐synacthen cortisol) should be considered similarly, but the SST is likely to be more reproducible. Low morning cortisol values are predictive of adrenal insufficiency, whereas high cortisol values indicate a low adrenal insufficiency risk; however, many patients have indeterminate values and an SST can be useful.26 The interpretation of the cortisol testing in this context is detailed in Box 3.
Development of symptoms in the supraphysiological dose range requires discernment whether they represent recrudescence of the underlying disease that led to glucocorticoid use or hormone withdrawal syndrome. A slowing of the rate of withdrawal is required if symptoms develop. Excessively slow glucocorticoid withdrawal can prolong adrenal suppression, increasing the risk of adrenal crisis, as seen after treatment for Cushing syndrome.29
In summary, individualised glucocorticoid weaning can reduce the risk of Cushingoid side effects and facilitate prompt restoration of adrenal function.
Box 1 – Physiological doses of glucocorticoid3
Type of glucocorticoid |
Dose per day | ||||||||||||||
Hydrocortisone |
15–25 mg |
||||||||||||||
Cortisone acetate |
20–30 mg |
||||||||||||||
Prednisolone |
4–6 mg |
||||||||||||||
Prednisone |
4–6 mg |
||||||||||||||
Dexamethasone |
0.4 mg |
||||||||||||||
Box 2 – The hypothalamic–pituitary–adrenal (HPA) axis consists of paraventricular hypothalamic nuclei producing corticotropin‐releasing hormone (CRH), secreted into a portal venous system to stimulate the pituitary corticotropes, which produce adrenocorticotropic hormone (ACTH), which acts to stimulate cortisol production and secretion*
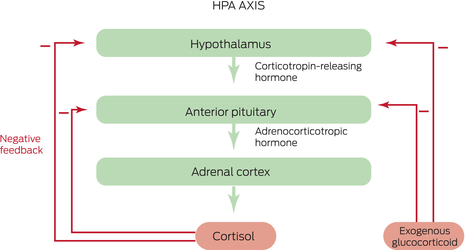
* Cortisol negative feedback inhibition suppresses CRH and ACTH secretion, via glucocorticoid receptors, to prevent hypercortisolism. Exogenous glucocorticoids act via this negative feedback mechanism to suppress endogenous CRH, ACTH and cortisol.
Box 3 – Interpretation of morning cortisol and actions suggested to wean glucocorticoid*
Morning plasma cortisol (nmol/L) |
Supraphysiological dose glucocorticoid |
Physiological dose glucocorticoid | |||||||||||||
< 80 |
Wean to physiological dose, 5 mg prednisolone per week or equivalent, while observing for disease recrudescence or hormone withdrawal syndrome |
|
|||||||||||||
81–400 |
Can wean to physiological dose over 6 weeks |
|
|||||||||||||
> 400‡ |
Weaning can be rapid to complete withdrawal over 2 weeks if taking a high dose or more quickly for near physiological doses |
|
|||||||||||||
SST = short synacthen test. * Interpretation of AM and stimulated cortisol in patients treated with glucocorticoid. † Biochemical cortisol monitoring — morning cortisol or SST may overdiagnose adrenal insufficiency in some cases and the need to use either, but especially SST, should consider the patient's risk and circumstances. SST may be needed to verify ongoing suppression at six‐month intervals. To convert cortisol from nmol/L to μg/dL, divide by 27.59. ‡ No adrenal suppression. | |||||||||||||||
Provenance: Commissioned; externally peer reviewed.
- 1. Wendler NL, Graber RP, Jones RE, Tishler M. Synthesis of 11‐hydroxylated cortical steroids: 17α hydroxycorticosterone. J Am Chem Soc 1950; 72: 5793‐5794.
- 2. Herzog HL, Nobile A, Jevnik MA, et al. Microbiological transformation of steroids. Science 1955; 121: 176.
- 3. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 364‐389.
- 4. Pofi R, Caratti D, Ray DW, Tomlinson JW. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr Rev 2023; https://doi.org/10.1210/endrev/bnad016 [Epub ahead of print].
- 5. Einarsdottir MJ, Ekman P, Trimpou P, et al. High prescription rate of oral glucocorticoids in children and adults: a retrospective cohort study from western Sweden. Clin Endocrinol (Oxf) 2020; 92: 21‐28.
- 6. Van Staa TP, Leufkens HGM, Abenhaim L, et al. Use of oral corticosteroids in the United Kingdom. QJM Mon J Assoc Physicians 2000; 93: 105‐111.
- 7. Laugesen K, Lunde Jorgensen JO, Petersen I, et al. Fifteen‐year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur J Endocrinol 2019; 181: 267‐273.
- 8. Volkmann ER, Rezai S, Tarp S, et al. We still don't know how to taper glucocorticoids in rheumatoid arthritis, and we can do better. J Rheumatol 2013; 40: 1646.
- 9. Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am 2002; 31: 751‐778.
- 10. Kehlet H, Binder C. Value of an ACTH test in assessing hypothalamic‐pituitary‐adrenocortical function in glucocorticoid‐treated patients. Br Med J 1973; 2: 147‐149.
- 11. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985; 117: 2505‐2511.
- 12. Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018; 21: 403‐416.
- 13. Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci 1995; 771: 1‐18.
- 14. Gomez MT, Magiakou MA, Mastorakos G, Chrousos GP. The pituitary corticotroph is not the rate limiting step in the postoperative recovery of the hypothalamic‐pituitary‐adrenal axis in patients with Cushing syndrome. J Clin Endocrinol Metab 1993; 77: 173‐177.
- 15. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med 2019; 381: 852‐861.
- 16. Rushworth RL, Chrisp GL, Torpy DJ. Glucocorticoid‐induced adrenal insufficiency: a study of the incidence in hospital patients and a review of peri‐operative management. Endocr Pract 2018; 24: 437‐445.
- 17. Theiler‐Schwetz V, Prete A. Glucocorticoid withdrawal syndrome: what to expect and how to manage. Curr Opin Endocrinol Diabetes Obes 2023; 30: 167‐174.
- 18. Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev 2003; 24: 523‐538.
- 19. Wiebka A, Pearce S, Simpson H. Surgical guidelines for Addison's disease and other forms of adrenal insufficiency. Addison's Clinical Advisor Panel. https://www.addisonsdisease.org.uk/surgery (viewed July 2023).
- 20. Hafner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 2015; 100: 407‐416.
- 21. Broersen LHA, van Haalen FM, Kienitz T, et al. The incidence of adrenal crisis in the postoperative period of HPA axis insufficiency after surgical treatment for Cushing's syndrome. Eur J Endocrinol 2019; 181: 201‐210.
- 22. Broersen LHA, Pereira AM, Jørgensen JOL, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta‐analysis. J Clin Endocrinol Metab 2015; 100: 2171‐2180.
- 23. Werumeus Bunning J, Touw DJ, Brummelman P, et al. Pharmacokinetics of oral hydrocortisone — results and implications from a randomized controlled trial. Metabolism 2017; 71: 7‐16.
- 24. Huang H, Wang W. Molecular mechanisms of glucocorticoid resistance. Eur J Clin Invest 2023; 53: e13901.
- 25. Esteban NV, Loughlin T, Yergey AI, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72: 39‐45.
- 26. Yo WS, Toh LM, Brown SJ, et al. How good is a morning cortisol in predicting an adequate response to intramuscular Synacthen stimulation? Clin Endocrinol (Oxf) 2014; 81: 19‐24.
- 27. El‐Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva — are our assays good enough? Ann Clin Biochem 2017; 54: 308‐322.
- 28. Lee JH, Meyer EJ, Nenke MA, et al. Corticosteroid‐binding globulin (CBG): spatiotemporal distribution of cortisol in sepsis. Trends Endocrinol Metab 2023; 34: 181‐190.
- 29. Torpy DJ. Commentary on article: adrenal crisis in treated patients with Cushing's syndrome. Eur J Endocrinol 2019; 181: C13‐C15.






Open access:
Open access publishing facilitated by The University of Adelaide, as part of the Wiley – The University of Adelaide agreement via the Council of Australian University Librarians.
No relevant disclosures.