The known: Consensus regarding screening for gestational diabetes mellitus (GDM) is limited. During the COVID‐19 pandemic, screening approaches were modified to reduce the need for pregnant women to attend clinics for oral glucose tolerance tests.
The new: In Australia, ruling out GDM in women with fasting blood glucose levels of less than 4.7 mmol/L during the first year of the COVID‐19 pandemic was not associated with increased frequencies of adverse perinatal outcomes for mothers or their children.
The implications: Fasting blood glucose assessment could be adequate for ruling out GDM in women at low absolute risk of glycaemia‐related pregnancy complications.
Gestational diabetes mellitus (GDM) is a common condition with short and long term implications for both mother and child. Perinatal risks include large for gestational age infants, greater likelihood of instrumental or caesarean delivery and labour induction,1,2 pre‐term birth, neonatal hypoglycaemia, and pre‐eclampsia.3 As appropriate management can reduce the risk of adverse outcomes,4,5,6 recognising and diagnosing GDM is important.
The International Association of Diabetes in Pregnancy Study Groups (IADPSG) updated their GDM screening and diagnostic recommendations in 2010,7 following the Hyperglycemia and Adverse Pregnancy outcomes (HAPO) study.1 The Australasian Diabetes in Pregnancy Society adopted the IADPSG recommendations in 2014.8 Most Australian health services, including Queensland Health, adopted the recommended screening process, in which all pregnant women undergo oral glucose tolerance tests (OGTTs) at 24–28 weeks of pregnancy.9 Some professional organisations, however, were opposed to the new recommendations.10
The coronavirus disease 2019 (COVID‐19) pandemic prompted health services across Australia to recommend a modified GDM screening procedure in late March 2020: fasting venous plasma glucose (FVPG) assessment at 24–28 weeks of pregnancy, followed by OGTTs for women with FVPG values of 4.7–5.0 mmol/L.11 Women with FVPG values below 4.7 mmol/L were empirically classified as not having GDM; the absolute risk of glycaemia‐related pregnancy complications appear to be low for women with FVPG values at this level, even when one‐ or two‐hour OGTT results are consistent with GDM.12 Women with FVPG values exceeding 5.0 mmol/L were treated for GDM.11
We examined the impact of changes to GDM screening and diagnosis in response to COVID‐19 on GDM diagnosis, maternal and infant outcomes, and screening process measures. Our aim was to determine whether ruling out GDM in women with FVPG values below 4.7 mmol/L was associated with perinatal outcomes similar to those following the standard (pre‐pandemic) OGTT procedure. We also compared maternal and infant outcomes for all pregnant women in 2019 and 2020, separately for women with or without GDM.
Methods
In our retrospective pre–post study, we compared outcomes for all women who gave birth in Queensland during 1 July – 31 December 2019 with those of women who gave birth during 1 July – 31 December 2020, based on data extracted from the Perinatal Data Collection (PDC) by the Queensland Health Statistical Services Branch.
GDM is coded in the PDC according to the International Classification of Diseases, tenth revision (ICD‐10; code O24). We sought to link the GDM status of each woman with OGTT and FVPG assessment results in the databases of the three major Queensland pathology companies, but barriers to linkage with private pathology company data meant that complete blood glucose data were available only for assessments by the public Pathology Queensland. While GDM status could be determined from ICD‐10 coding, diagnostic methods could only be determined for women assessed by Pathology Queensland.
Deterministic and probabilistic record linkage was undertaken and a de‐identified linked data extract provided for our analysis by the Queensland Health Statistical Services Branch. Record groups with uncertain probabilistic linkage matches (0.2–0.8 probability) were individually reviewed.
Available maternal characteristics data for the two study periods are summarised as descriptive statistics. Although we could not link individual GDM status with private pathology OGTT and FVPG results, data for all OGTTs for pregnant women in Queensland during the two study periods were available; we summarise these results as descriptive statistics. We determined how many women were classified as having or not having GDM during the second half of 2020 from the available FVPG and OGTT data. We categorised women as having or not having GDM according to ICD‐10 coding, and grouped them by diagnostic method, when known: OGTT and glycated haemoglobin (HbA1c) assessment, glucose data unavailable, or FVPG assessment (2020 only).
We used a classification tree to compare data for all women (regardless of whether glucose data were available) to identify any differences and sources of bias related to missing data that could influence our interpretation of results (Supporting Information).
Outcomes
The maternal outcomes we examined were gestational hypertension (including pre‐eclampsia, haemolysis, elevated liver enzyme levels, and low platelet count), caesarean delivery, birthweight, and pre‐term delivery (before 37 weeks’ gestation). The neonatal outcomes we examined were large for gestational age (birthweight beyond the 90th percentile13), small for gestational age (birthweight below the 10th percentile13), hypoglycaemia (blood glucose level below 2.6 mmol/L), and respiratory distress.
Statistical analyses
We undertook three analyses. We compared women for whom GDM was excluded in 2019 on the basis of OGTT or HbA1c results and women for whom it was excluded in 2020 on the basis of FVPG assessment alone. We then compared women for whom GDM was excluded in 2019 and 2020, excluding those for whom FVPG testing was undertaken in 2020. Finally, we compared perinatal complications for women who were diagnosed with GDM (ICD‐10 code O24) in 2019 and 2020.
For these comparisons, we used Gaussian multiple variable regression models, adjusted for confounders according to the findings of our previous study.14 Our large sample size facilitated safely applying a Gaussian model to binary data.15 The models included a random intercept for each mother to control for women giving birth in both half‐years. The models were fitted in a Bayesian paradigm. For binary outcomes, we report mean differences in probability with 95% credible intervals (CrIs), and for continuous outcomes mean proportional changes with 95% CrIs. We also report estimated absolute changes as numbers per 1000 births.
Confinement dates were reported in the dataset as month and year, but as we required exact dates to merge them with other data we used multiple imputation to randomly assign a day to each mother's confinement. We fitted regression models to each of ten generated imputed datasets, from which we derived the combined estimates reported.
All models were fitted using R 4.20 (R Foundation for Statistical Computing) and the R package nimble 0.12.2.16
Ethics approval
The human research ethics committee of the Royal Brisbane and Women's Hospital (LNR/2020/QRBW/72113) approved the study. The Director‐General of Queensland Health waived the requirement for individual consent to the release of confidential information under the Public Health Act (Qld) 2005.
Results
A total of 29 113 pregnancies were recorded in Queensland during 1 July – 31 December 2019, and 28 778 during 1 July – 31 December 2020; 3968 women (13.6%) were diagnosed with GDM in 2019, 4029 (14.0%) in 2020 (Box 1). In 2020, FVPG assessments established GDM in 216 women (1.1%) and excluded it in 1660 (5.8%). In 2019, glucose data were not available for 2480 women with GDM (8.5%) and 18 848 who did not have GDM (65%); in 2020, glucose data were not available for 2811 women with GDM (9.8%) and 20 070 who did not have GDM (70%). A total of 31 827 OGTTs for pregnant women were undertaken by the three major Queensland pathology services in 2019, and 23 862 in 2020 (25% fewer) (Box 2).
Glucose data were available for 6297 women not diagnosed with GDM in 2019 and for 1660 in 2020 (Box 2). Glucose data were unavailable for larger proportions of women who attended private rather than public antenatal facilities (0.94 v 0.65). Among women receiving public antenatal care, glucose data were unavailable for a larger proportion of those who lived in major cities than for those living elsewhere (0.76 v 0.48), and for larger proportions of those who had fewer than nine antenatal care visits than for those who had nine or more visits (major cities: 0.83 v 0.72; outside major cities: 0.60 v 0.44) (Supporting Information, figure 1).
Perinatal outcomes for women not diagnosed with gestational diabetes mellitus
Outcomes were generally similar for the 6297 women for whom GDM was excluded in 2019 on the basis of OGTT or HbA1c results and the 1660 women for whom it was excluded in 2020 on the basis of FVPG assessment alone. The exception was caesarean delivery: the estimated probability increase in 2020 was 3.9 percentage points (95% CrI, 2.2–5.6 percentage points) (Box 3), corresponding to an extra 6.8 caesarean deliveries per 1000 births, increasing from 166 to 172.8 per 1000 births (Box 4).
The probabilities of several outcomes were higher for the 23 089 women without GDM in 2020 (excluding those diagnosed on the basis of FVPG assessment alone) than for the 25 145 women without GDM in 2019: respiratory distress (1.0 percentage points; 95% CrI, 0.5–1.4 percentage points), neonatal intensive care or special nursery admission (0.9 percentage points; 95% CrI, 0.2–1.6 percentage points), caesarean delivery (2.0 percentage points; 95% CrI, 1.3–2.7 percentage points), and large for gestational age babies (1.0 percentage points; 95% CrI, 0.4–1.5 percentage points). Mean birthweight was 0.76% greater in 2020 (95% CrI, 0.43–1.08%) (Box 5).
Perinatal outcomes for women diagnosed with gestational diabetes mellitus
Among women diagnosed with GDM (3968 in 2019, 4029 in 2020), mean infant birthweight was higher in 2020 than 2019 (mean proportional change, 1.6%; 95% CrI, 0.81–2.3%); the probability of large for gestational age infants (mean probability difference, 1.9 percentage points; 95% CrI, 0.4–3.3 percentage points), that of small for gestational age infants lower (–1.1 percentage points; 95% CrI, –0.1 to –2.0 percentage points) (Box 6). However, the caesarean delivery rate was not higher in 2020 (Box 7), nor were the differences in the probability or number of neonatal intensive care unit or special care nursery admissions statistically significant. We could not compare outcomes for women diagnosed with GDM in 2019 with those of women diagnosed in 2020 on the basis of FVPG assessment alone, given the small proportion of women in the latter group (1.1%; Box 2).
Discussion
We investigated whether maternal and infant perinatal outcomes following a modified GDM screening procedure in which GDM was excluded on the basis of an FVPG value below 4.7 mmol/L were similar to outcomes following the standard OGTT diagnostic procedure. FVPG assessment was a pragmatic option during the COVID‐19 pandemic, and this period provided an opportunity to examine whether it could be safely used as a screening tool for women with a low absolute risk of adverse outcomes. We found that rates of adverse outcome were similar using the two methods.
Australian studies have found that FVPG values above 4.6 mmol/L reasonably predict OGTT results diagnostic for GDM — our retrospective evaluation of 26 683 OGTTs from the first half of 2015 indicated that 95% of women could be identified as having GDM on the basis of high FVPG values17 — and which women will require pharmacotherapy for GDM.18 Similarly, a post hoc HAPO analysis found that women not treated for GDM who had FVPG values below 4.6 mmol/L experienced fewer complications than women with higher values.12 Assessing FVPG as the first step in GDM screening could be an alternative to universal OGTT screening, reducing the number of OGTTs required by 50–80%.12,17
We found that the frequency of most perinatal outcomes for women in whom GDM was ruled out by an FVPG value below 4.7 mmol/L in 2020 were similar to those for women without GDM in 2019; the exception was that caesarean deliveries were slightly more frequent in 2020. As the results were similar when we compared outcomes for all women without GDM in 2019 and 2020 (except those diagnosed on the basis of FVPG alone), the differences were probably related to the impact of the COVID‐19 pandemic on maternity care during 2020. Although Queensland was relatively COVID‐19‐free during 2020, lockdowns and restrictions on movements affected antenatal health care delivery. Reduced physical activity,19 increased stress and anxiety,19 and gestational weight gain20 during the pandemic have been reported and probably influenced outcomes. Increased numbers of caesarean deliveries during the first year of the COVID‐19 pandemic were reported overseas.21,22,23
GDM screening and diagnosis recommendations were modified in many countries during the COVID‐19 pandemic.24 Prior to the pandemic, different GDM diagnostic criteria were applied in Canada, Australia, and the United Kingdom. During the pandemic, HbA1c values exceeding 5.7% (39 mmol/mol) were sufficient for a GDM diagnosis in both Canada and the United Kingdom, as were FVPG values of 5.6 mmol/L or random venous plasma glucose values of 9.0 mmol/L (United Kingdom) or 11.1 mmol/L (Canada).24 An evaluation of the three national approaches on the basis of HAPO data found that the modified criteria would markedly reduce the number of GDM diagnoses in the United Kingdom (by 81%) and Canada (by 82%), but only by 25% in Australia.24 The frequency of most adverse outcomes, including pre‐term birth, large for gestational age babies, primary caesarean deliveries, and neonatal hyperinsulinemia, increased significantly among women in the United Kingdom and Canada in whom GDM was initially excluded but later diagnosed, but not in Australia.24 A more recent retrospective evaluation found a slight increase in the number of GDM diagnoses in Australia during the COVID‐19 pandemic; 50% of women were diagnosed according to the modified COVID‐19 criteria; the rates of most outcomes did not change, apart from increases in the frequency of instrumental deliveries and shoulder dystocia.25 In our study, undiagnosed GDM may have contributed to the slightly increased probability of respiratory distress for the babies of women without GDM in 2020.
Limitations
A major limitation was the large proportion of missing glucose data, as we could not link private pathology company data with outcomes. Despite approval for data linkage, concerns about patient privacy and the lack of processes for data sharing by health care organisations precluded negotiation of an acceptable data linkage process. However, a study that compared OGTT results during 2013–2015 found that differences between the three main pathology providers in reported mean results at 0, 60, and 120 minutes were negligible.17
Glucose data were more frequently unavailable for women using private maternity care or living in major cities, probably because of greater access to private pathology providers. Not having these data may have influenced our findings. Despite rates of gestational hypertension and GDM in women receiving private or public care being similar, those receiving private care are more likely to have caesarean deliveries and their babies are less likely to be admitted to neonatal intensive care or special care nurseries.26 However, the mean pre‐pregnancy body mass index of women receiving private care is lower and their mean age higher than for those receiving public care in Australia,27 and both factors influence the likelihood of being diagnosed with GDM.
Our inability to include private pathology service glucose data should therefore be considered when interpreting our findings. Strategies for overcoming this barrier to routine data‐based decisions and evaluations would more generally assist researchers, clinicians, and decision makers investigate similar research questions.28
Conclusions
The discussions of GDM screening and diagnosis in several countries suggest that an international consensus is unlikely. However, we need to reduce the burden and costs of testing by selecting a method that identifies women who are at lower risk of adverse perinatal outcomes. The COVID‐19 pandemic facilitated a natural experiment in which different screening and diagnostic recommendations could be examined. Using FVPG assessment to identify women at low absolute risk of GDM‐related pregnancy complications as an initial step in GDM screening could benefit a large proportion of pregnant women and save the health system substantial costs.
Box 1 – Characteristics of Queensland women tested for gestational diabetes mellitus, 1 July – 31 December 2019 and 1 July – 31 December 2020, and of their infants, by infant's year of birth
|
Characteristic |
1 July – 31 December 2019 |
1 July – 31 December 2020 |
|||||||||||||
|
|
|||||||||||||||
|
Women |
29 113 |
28 778 |
|||||||||||||
|
Gestational diabetes diagnosis |
3968 (13.6%) |
4029 (14.0%) |
|||||||||||||
|
Age (years), median (IQR) |
31 (27–34) |
31 (27–34) |
|||||||||||||
|
Pre‐pregnancy body mass index (kg/m2), median (IQR) |
25 (22–29) |
25 (22–29) |
|||||||||||||
|
Missing data |
268 |
210 |
|||||||||||||
|
Indigenous status |
|
|
|||||||||||||
|
Indigenous |
2123 (7.3%) |
2143 (7.4%) |
|||||||||||||
|
Non‐Indigenous |
26 990 (92.7%) |
26 605 (92%) |
|||||||||||||
|
Missing data |
0 |
30 (0.1%) |
|||||||||||||
|
Ever smoked |
3319 (11.4%) |
3212 (11.2%) |
|||||||||||||
|
Previous Caesarean delivery |
5270 (18.1%) |
5207 (18.1%) |
|||||||||||||
|
Missing data |
0 |
180 |
|||||||||||||
|
Parity, median (IQR) |
1 (0–2) |
1 (0–2) |
|||||||||||||
|
Infants |
29 581 |
29 219 |
|||||||||||||
|
Gestation (weeks), median (IQR) |
39 (38–40) |
39 (38–40) |
|||||||||||||
|
Birthweight (g), median (IQR) |
3370 (3027–3700) |
3384 (3040–3710) |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
Box 2 – Assessment by three major pathology services of Queensland women for gestational diabetes mellitus, 1 July – 31 December 2019 and 1 July – 31 December 2020, by outcome and method
|
Characteristic |
1 July – 31 December 2019 |
1 July – 31 December 2020 |
|||||||||||||
|
|
|||||||||||||||
|
Total number of women |
29 113 |
28 778 |
|||||||||||||
|
Women with gestational diabetes mellitus |
3968 (13.6%) |
4029 (14.0%) |
|||||||||||||
|
Oral glucose tolerance test or HbA1c assessment |
1488 (5.1%) |
902 (3.1%) |
|||||||||||||
|
Glucose data unavailable |
2480 (8.5%) |
2811 (9.8%) |
|||||||||||||
|
Fasting blood glucose only |
— |
316 (1.1%) |
|||||||||||||
|
Women with no gestational diabetes mellitus |
25 145 (86.4%) |
24 749 (86.0%) |
|||||||||||||
|
Oral glucose tolerance test or HbA1c assessment |
6297 (21.6%) |
3019 (10.5%) |
|||||||||||||
|
Glucose data unavailable |
18 848 (64.7%) |
20 070 (69.7%) |
|||||||||||||
|
Fasting blood glucose only |
— |
1660 (5.8%) |
|||||||||||||
|
Oral glucose tolerance tests for pregnant women |
31 827 |
23 862 |
|||||||||||||
|
Pathology Queensland |
9868 |
8870 |
|||||||||||||
|
Private pathology service 1 |
11 460 |
8719 |
|||||||||||||
|
Private pathology service 2 |
10 499 |
6273 |
|||||||||||||
|
|
|||||||||||||||
|
HbA1c = glycated haemoglobin. |
|||||||||||||||
Box 3 – Mean differences in perinatal outcomes for women not diagnosed with gestational diabetes mellitus, 1 July – 31 December 2020 (fasting venous plasma glucose assessment only) v 1 July – 31 December 2019 (oral glucose tolerance testing)*
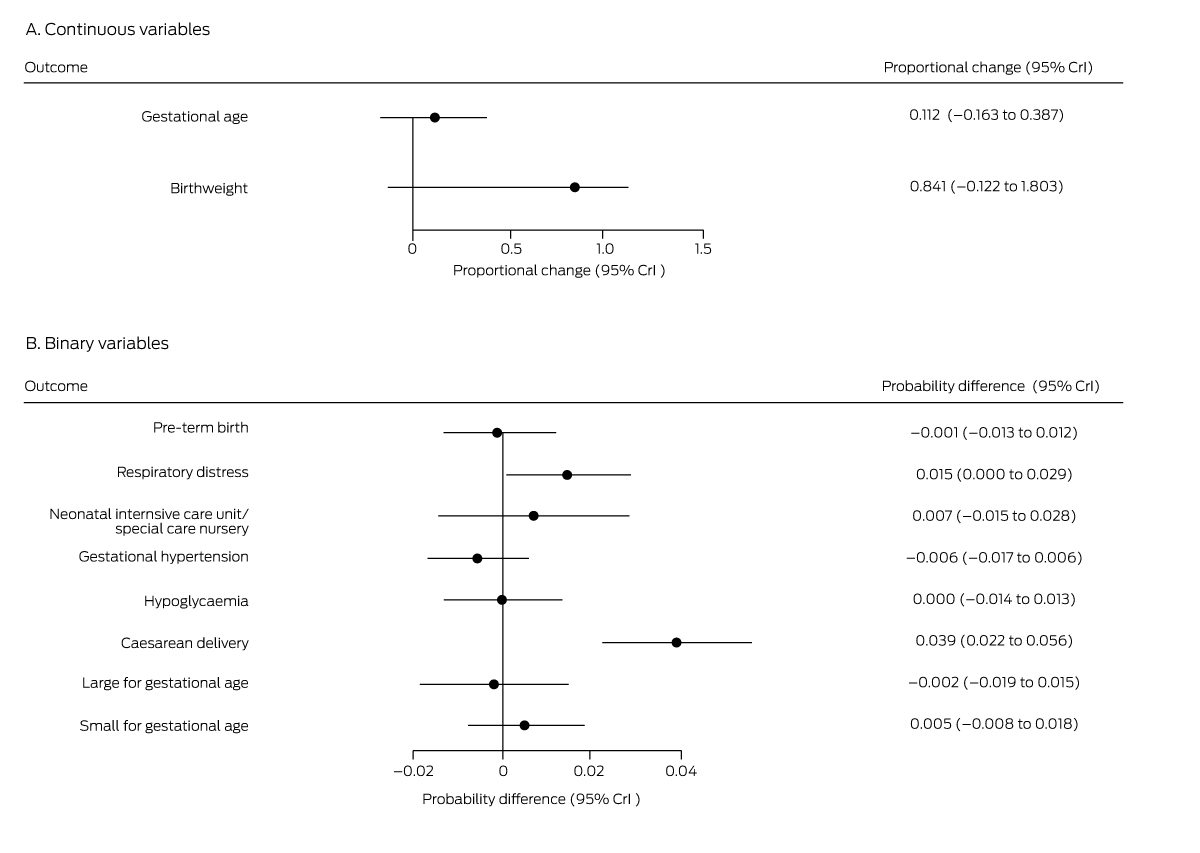
CrI = credible interval. * Derived from multiple regression models.
Box 4 – Absolute risk of perinatal outcomes for women women not diagnosed with gestational diabetes mellitus, 1 July – 31 December 2020 (fasting venous plasma glucose assessment only) or 1 July – 31 December 2019 (oral glucose tolerance testing): number per 1000 births (95% credible interval)*
|
Outcome |
1 July – 31 December 2019 |
1 July – 31 December 2020 |
|||||||||||||
|
|
|||||||||||||||
|
Small for gestational age |
74.2 |
74.5 (73.6–75.5) |
|||||||||||||
|
Large for gestational age |
110 |
110 (108–112) |
|||||||||||||
|
Caesarean delivery |
166 |
172.8 (170–176) |
|||||||||||||
|
Gestational hypertension |
57.5 |
57.2 (56.5–57.8) |
|||||||||||||
|
Hypoglycaemia |
79.8 |
79.8 (78.8–80.9) |
|||||||||||||
|
Neonatal intensive care unit admission |
201 |
202.5 (198–207) |
|||||||||||||
|
Respiratory distress |
75.1 |
76.2 (75.2–77.3) |
|||||||||||||
|
Pre‐term birth (< 37 weeks) |
91.1 |
91.0 (89.8–92.1) |
|||||||||||||
|
|
|||||||||||||||
|
* Derived from multiple regression models. |
|||||||||||||||
Box 5 – Mean differences in perinatal outcomes for women not diagnosed with gestational diabetes mellitus, 1 July – 31 December 2020 (any method except fasting venous plasma glucose assessment) v 1 July – 31 December 2019 (oral glucose tolerance testing)*
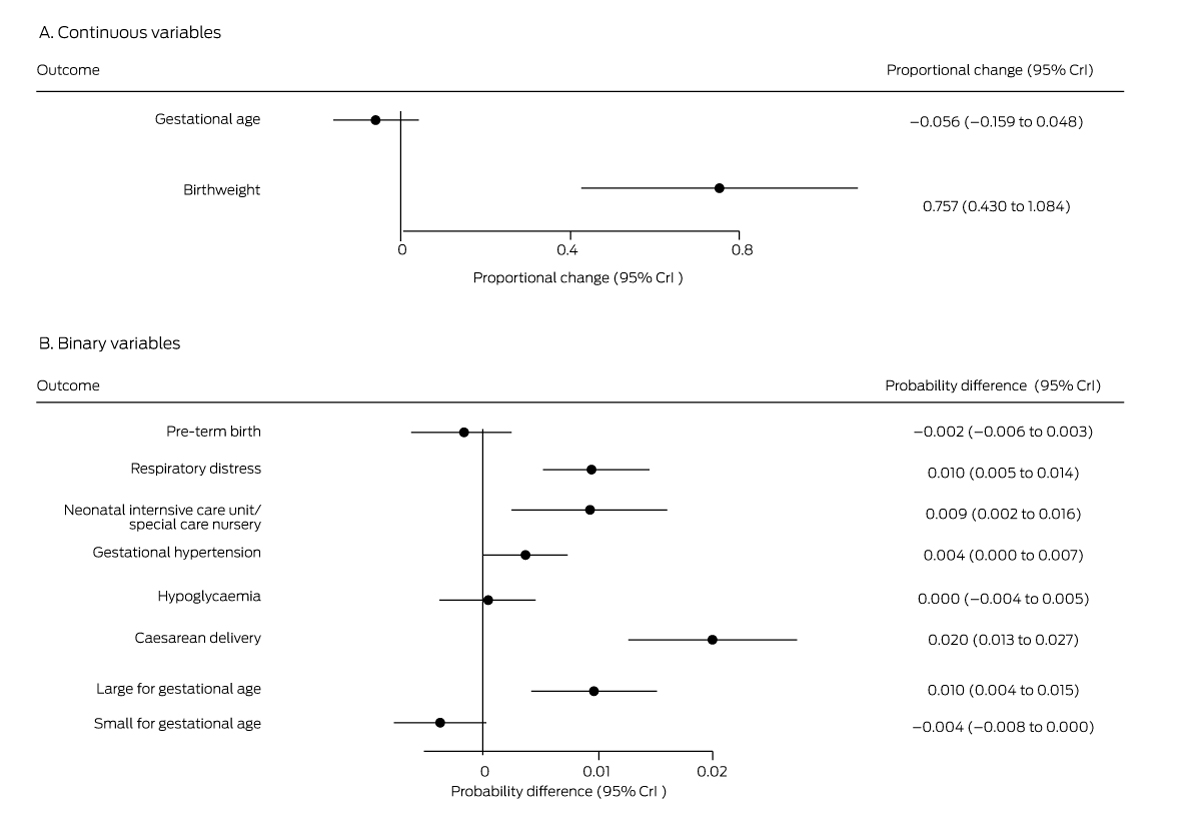
* Derived from multiple regression models.
Box 6 – Mean differences in perinatal outcomes for women diagnosed with gestational diabetes mellitus, 1 July – 31 December 2020 (any method) v 1 July – 31 December 2019 (oral glucose tolerance testing)*
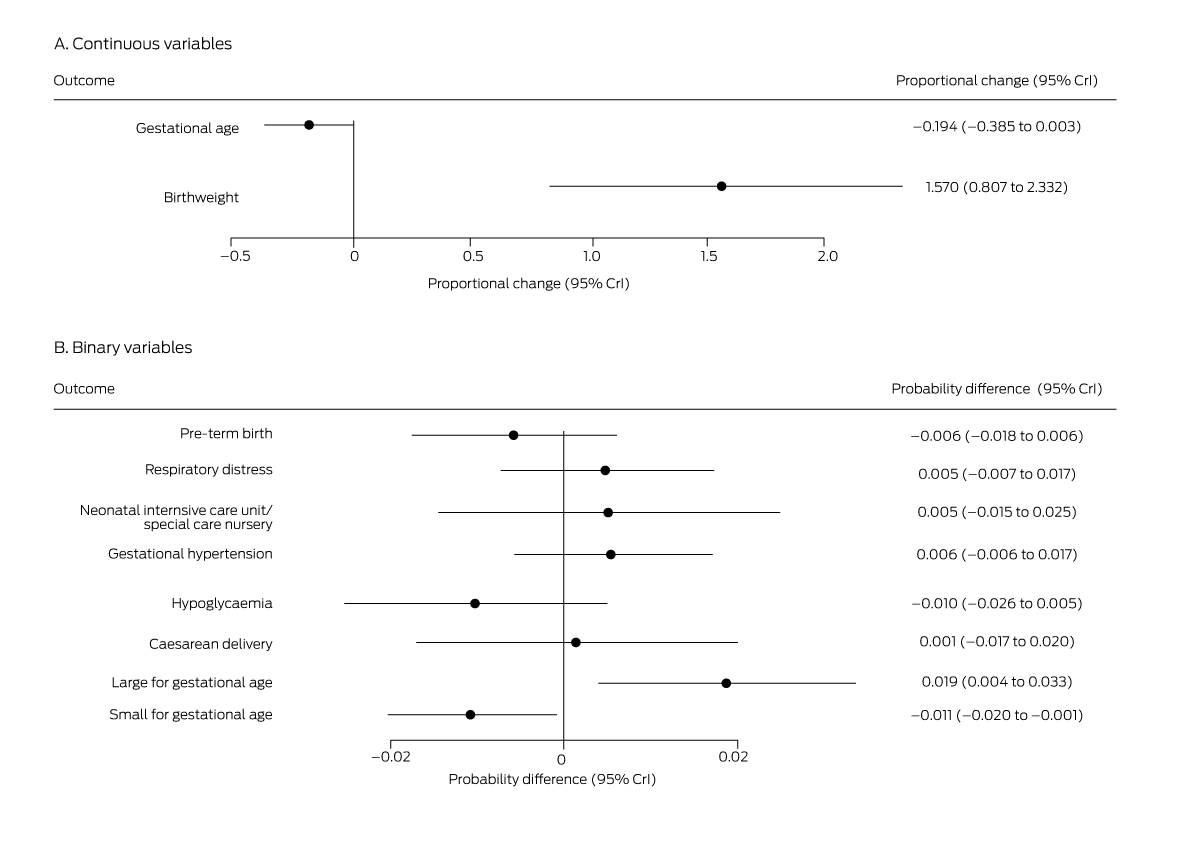
* Derived from multiple regression models.
Box 7 – Estimated absolute risk of perinatal outcomes for women with gestational diabetes mellitus, 1 July – 31 December 2020 (any method) or 1 July – 31 December 2019: number per 1000 births (95% credible interval)*
|
Outcome |
1 July – 31 December 2019 |
1 July – 31 December 2020 |
|||||||||||||
|
|
|||||||||||||||
|
Small for gestational age |
59.9 |
59.2 (58.7–59.8) |
|||||||||||||
|
Large for gestational age |
135 |
137 (135–139) |
|||||||||||||
|
Caesarean delivery |
439.4 |
440 (432–448) |
|||||||||||||
|
Gestational hypertension |
77.1 |
77.6 (76.7–78.4) |
|||||||||||||
|
Hypoglycaemia |
145.0 |
144 (141–146) |
|||||||||||||
|
Neonatal intensive care unit admission |
281.6 |
283 (278–289) |
|||||||||||||
|
Respiratory distress |
86.8 |
87.2 (86.1–88.2) |
|||||||||||||
|
Pre‐term birth (< 37 weeks) |
101 |
101 (99.6–102) |
|||||||||||||
|
|
|||||||||||||||
|
* Derived from multiple regression models. |
|||||||||||||||
Received 16 February 2023, accepted 10 July 2023
- Nina JL Meloncelli1
- Adrian G Barnett2
- Cate M Cameron2,3
- David McIntyre4
- Leonie K Callaway5
- Michael C d'Emden5
- Susan J de Jersey1,5
- 1 Centre for Health Services Research, the University of Queensland, Brisbane, QLD
- 2 Australian Centre for Health Services Innovation and Centre for Healthcare Transformation, Queensland University of Technology, Brisbane, QLD
- 3 Jamieson Trauma Institute, Royal Brisbane and Women's Hospital, Metro North Health, Brisbane, QLD
- 4 Mater Research, the University of Queensland, Brisbane, QLD
- 5 Royal Brisbane and Women's Hospital, Metro North Health, Brisbane, QLD
Open access:
Open access publishing facilitated by The University of Queensland, as part of the Wiley – The University of Queensland agreement via the Council of Australian University Librarians.
We acknowledge Charles Appleton and Julia Chang (QML Pathology), David Kanowski (Sullivan and Nicolaides Pathology) and Michael Marks (Queensland Health Statistical Services Branch) for providing oral glucose tolerance testing numbers, and their assistance with attempting to link private pathology and perinatal data. Susan de Jersey is supported by a Metro North Health Clinician Research Fellowship. This study was funded by a Royal Brisbane and Women’s Hospital Foundation special COVID-19 Grant. The funding body has no role in the planning, writing or publication of the work or any role in study design, data collection, analysis, interpretation, reporting or publication.
No relevant disclosures.
- 1. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991‐2002.
- 2. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014; 103: 341‐363.
- 3. Venkatesh KK, Lynch CD, Powe CE, et al. Risk of adverse pregnancy outcomes among pregnant individuals with gestational diabetes by race and ethnicity in the United States, 2014–2020. JAMA 2022; 327: 1356‐1367.
- 4. American Diabetes Association Professional Practice Committee. Management of diabetes in pregnancy. Standards of medical care in diabetes: 2022. Diabetes Care 2022; 45 (Suppl 1): S232‐S243.
- 5. Crowther CA, Hiller JE, Moss JR, et al; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477‐2486.
- 6. Landon MB, Spong CY, Thom E, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339‐1348.
- 7. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676‐682.
- 8. Nankervis A, McIntyre HD, Moses R, et al; Australasian Diabetes in Pregnancy Society. ADIPS consensus guidelines for the testing and diagnosis of hyperglycaemia in pregnancy in Australia and New Zealand. Nov 2014. https://www.adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014_000.pdf (viewed Mar 2022).
- 9. Meloncelli N, Barnett A, Pelly F, de Jersey S. Diagnosis and management practices for gestational diabetes mellitus in Australia: cross‐sectional survey of the multidisciplinary team. Aust N Z J Obstet Gynaecol 2019; 59: 208‐214.
- 10. Nankervis A, Conn J. Gestational diabetes mellitus: negotiating the confusion. Aust Fam Physician 2013; 42: 528‐531.
- 11. Queensland Health. GDM screening and testing when local risk of COVID‐19 is elevated. 2020. https://www.health.qld.gov.au/__data/assets/pdf_file/0024/950505/f‐gdm‐diagnosis‐covid.pdf (viewed Mar 2022).
- 12. McIntyre H, Gibbons KS, Sacks DA, et al. Using fasting plasma glucose to identify women with gestational diabetes at low risk of complications [abstract]. 55th EASD Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20 September 2019. Diabetologia 2019; 62 (Suppl 1): S79.
- 13. Fenton TR, Kim JH. A systematic review and meta‐analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics 2013; 13: 59.
- 14. Meloncelli NJL, Barnett AG, D'Emden M, De Jersey SJ. Effects of changing diagnostic criteria for gestational diabetes mellitus in Queensland, Australia. Obstet Gynecol 2020; 135: 1215‐1221.
- 15. Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health 2002; 23: 151‐169.
- 16. de Valpine P, Turek D, Paciorek CJ, et al. Programming with models: writing statistical algorithms for general model structures with NIMBLE. J Comput Graph Stat 2017; 26: 403‐413.
- 17. d'Emden M, McLeod D, Ungerer J, et al. Development of a fasting blood glucose‐based strategy to diagnose women with gestational diabetes mellitus at increased risk of adverse outcomes in a COVID‐19 environment. PLoS One 2020; 15: e0243192.
- 18. Rodrigo N, Randall D, Al‐Hial FA, et al. Fasting glucose level on the oral glucose tolerance test is associated with the need for pharmacotherapy in gestational diabetes mellitus. Nutrients 2023; 15: 1226.
- 19. Stanton R, To QG, Khalesi S, et al. Depression, anxiety and stress during COVID‐19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health 2020; 17: 4065.
- 20. Cao W, Sun S, Danilack VA. Analysis of gestational weight gain during the COVID‐19 pandemic in the US. JAMA Network Open 2022; 5: e2230954.
- 21. Xue RH, Li J, Chen L, et al. Alternations of cesarean section rates in a non‐infected population after the outbreak of COVID‐19: a cross‐sectional study. Psychol Health Med 2022; 27: 1877‐1883.
- 22. Debrabandere ML, Farabaugh DC, Giordano C. A review on mode of delivery during COVID‐19 between December 2019 and April 2020. Am J Perinatol 2020; 38: 332‐341.
- 23. Elsaddig M, Khalil A. Effects of the COVID pandemic on pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol 2021; 73: 125‐136.
- 24. McIntyre HD, Gibbons KS, Ma RCW, et al. Testing for gestational diabetes during the COVID‐19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract 2020; 167: 108353.
- 25. Walker B, Edey J, Hall L, et al. Impact of new diagnostic pathway for gestational diabetes in time of COVID‐19. Obstet Med 2023; 16: 104‐108.
- 26. Robson SJ, Laws P, Sullivan EA. Adverse outcomes of labour in public and private hospitals in Australia: a population‐based descriptive study. Med J Aust 2009; 190: 474‐477. https://www.mja.com.au/journal/2009/190/9/adverse‐outcomes‐labour‐public‐and‐private‐hospitals‐australia‐population‐based
- 27. Callander E, Shand A, Ellwood D, et al. Financing maternity and early childhood healthcare in the Australian healthcare system: costs to funders in private and public hospitals over the first 1000 days. Int J Health Policy Manag 2021; 10: 554‐563.
- 28. Duszynski KM, Pratt NL, Lynch JW, et al; Vaccine Assessment Using Linked Data (VALiD) Working Group. Process trumps potential public good: better vaccine safety through linked cross‐jurisdictional immunisation data in Australia. Aust N Z J Public Health 2019; 43: 496‐503.






Abstract
Objective: To determine whether perinatal outcomes after excluding gestational diabetes mellitus (GDM) on the basis of fasting venous plasma glucose (FVPG) assessment during the coronavirus disease 2019 (COVID‐19) pandemic in 2020 were similar to those during the preceding year after excluding GDM using the standard oral glucose tolerance test (OGTT) procedure.
Design: Retrospective pre–post study.
Setting, participants: All women who gave birth in Queensland during 1 July – 31 December 2019 and 1 July – 31 December 2020.
Main outcome measures: Perinatal (maternal and neonatal) outcomes for pregnant women assessed for GDM, by assessment method (2019: OGTT/glycated haemoglobin [HbA1c] assessment; 2020: GDM could be excluded by an FVPG value below 4.7 mmol/L).
Results: 3968 of 29 113 pregnant women in Queensland during 1 July – 31 December 2019 (13.6%) were diagnosed with GDM, and 4029 of 28 778 during 1 July – 31 December 2020 (14.0%). In 2020, FVPG assessments established GDM in 216 women (1.1%) and excluded it in 1660 (5.8%). The frequencies of most perinatal outcomes were similar for women without GDM in 2019 and those for whom it was excluded in 2020 on the basis of FVPG values; the exception was caesarean delivery, for which the estimated probability increase in 2020 was 3.9 percentage points (95% credibility interval, 2.2–5.6 percentage points), corresponding to an extra 6.5 caesarean deliveries per 1000 births. The probabilities of several outcomes — respiratory distress, neonatal intensive care or special nursery admission, large for gestational age babies — were about one percentage point higher for women without GDM in 2020 (excluding those diagnosed on the basis of FVPG assessment alone) than for women without GDM in 2019.
Conclusions: Identifying women at low absolute risk of gestational diabetes‐related pregnancy complications on the basis of FVPG assessment as an initial step in GDM screening could reduce the burden for pregnant women and save the health system substantial costs.