In Australia, there are over 2 million elective admissions into hospital each year for major non-cardiac surgery, and this number is rising. Although these operations improve symptoms and reduce premature mortality, they come with risks, of which cardiovascular complications are the most frequent, making the management of peri-operative cardiovascular risk and events a common and growing burden for health services.1,2
Peri-operative major adverse cardiovascular events (MACE) are estimated to occur in about 3% of patients undergoing major non-cardiac surgery, and accounts for one-third of deaths at 30 days.3 Peri-operative MACE are regarded as those that occur within 30 days following surgery and encompass exacerbation or decompensation of existing cardiovascular disease (CVD) and first presentations of CVD, including ischaemic heart disease, stroke and transient ischaemic attack, arrhythmias, heart failure, cardiac arrest, and cardiovascular death.4 In addition, myocardial injury after non-cardiac surgery (MINS), defined as myocardial injury within 30 days after surgery (denoted by elevated troponin above the 99th percentile of the upper reference level for the troponin assay, with a rise/fall pattern), occurs in as many as 20% of patients and is a strong marker of future MACE.5
Accurate peri-operative risk assessment is important to enable shared decision making and to optimise the multidisciplinary management of patients undergoing major surgery, defined as a procedure necessitating overnight hospital stay.6 This narrative review aims to examine the evaluation of cardiovascular risk, as well as the prevention and management of cardiac complications in patients undergoing major non-cardiac surgery.
Methods
We searched the MEDLINE database to identify relevant articles on peri-operative cardiovascular risk management, using the keywords “pre-operative”, “peri-operative”, “major non-cardiac surgery”, “cardiovascular risk assessment”, “major adverse cardiovascular events”, “MACE”, “myocardial injury after non-cardiac surgery” and “MINS”, with the most recent search done in October 2022. We also identified relevant guidelines and reviews, including the guidelines from the Canadian Cardiovascular Society (CCS),6 the American College of Cardiology/American Heart Association (ACC/AHA),7 and the European Society of Cardiology/European Society of Anaesthesiology (ESC/ESA),8 and reviewed their reference lists.
Pre-operative cardiovascular risk assessment
There is considerable variation in peri-operative cardiovascular risk among patients presenting for surgery. For example, the peri-operative MACE rate is higher in adults aged 75 years and older compared with younger adults (9.5% v 4.8% respectively) and in patients with coronary stents compared to those without (9.5% v 1.5% respectively).9,10 Regardless of their pre-existing risk profile, patients who require urgent (within six to 24 hours) or emergency (within six hours) surgery are at an increased risk of peri-operative MACE.11 The level of cardiovascular risk is also a function of the proposed type and extent of surgery, with cataract and cosmetic surgery associated with less than 1% risk of peri-operative MACE, and peripheral vascular, thoracic and transplant surgery associated with a 5% risk or greater (Box 1).3,7,14
Pre-operative cardiovascular risk assessment is generally warranted in patients undergoing major elective non-cardiac surgery, with some guidelines specifically recommending this for all patients aged 45 years or older, or aged 18–44 years if they have a history of CVD. The extent of pre-operative cardiovascular risk assessment is often additionally informed by surgical factors, including the type of procedure, anaesthesia method, and the urgency of surgery.6
Clinical risk assessment
For all patients undergoing surgery, the pre-operative cardiovascular assessment starts with a history and examination. In both urgent and elective surgery settings, this is focused on identifying unstable cardiovascular conditions that substantially elevate a patient's risk of undergoing surgery and are considered contraindications to surgery, including acute coronary syndrome, decompensated heart failure, haemodynamically or clinically significant tachyarrhythmias or bradyarrhythmias, symptomatic severe valvular disease, or severe pulmonary hypertension.6,10 Specific symptoms that may elicit these unstable conditions include a history of exertional angina, dyspnoea, orthopnoea or recent syncope. Furthermore, the medical history should evaluate the presence of known cardiovascular conditions, including ischaemic heart disease, prior percutaneous coronary intervention, heart failure, valvular heart disease, arrhythmias, systemic or pulmonary hypertension, and risk factors for CVD, including diabetes or chronic kidney disease.14
Clinical examination should include assessment of haemodynamic status and examination for severe valvular disease or heart failure.10 Examination should be supplemented with a 12-lead electrocardiogram (ECG) for patients with suspected new heart disease or exacerbation of known ischaemic heart disease. ECG is also recommended in some guidelines for all patients with known CVD or cardiovascular risk factors, or with signs or symptoms of CVD before intermediate or high risk major surgeries, but it is acknowledged that the evidence base for these recommendations is weak.7,8 That is, pre-operative ECG abnormalities have not been consistently shown to predict peri-operative MACE in observational studies.16,17,18
Poor functional capacity, defined as an inability to perform four or more metabolic equivalents of task (roughly the equivalent of being unable to walk up a hill or climb two or more flights of stairs due to symptomatic limitation), is independently associated with a twofold increased risk of peri-operative complications.8,19 Cardiopulmonary exercise testing is the current gold standard for functional capacity assessment but is time and resource intensive and not widely available.20 Self-reported functional capacity has inconsistently been shown to predict peri-operative cardiovascular events.19,21,22,23 Therefore, screening tools such as the Duke Activity Status Index (DASI) are used to identify individuals requiring formal assessment. The DASI has shown to be an independent predictor of death or myocardial infarction.7,24 It gives a maximum score of 58.2, and a score below 34 is a threshold that denotes elevated cardiovascular risk based on observational evidence.25 A simplified version of the 12-part DASI questionnaire is the modified DASI (M-DASI-4Q) consisting of four questions (Box 2). The DASI and the M-DASI-4Q have been shown in observational studies to delineate those with and without satisfactory functional capacity, defined as an anaerobic threshold greater than 11 mL/kg per minute and oxygen consumption (VO2) peak greater than 16 mL/kg per minute.26,27 In scenarios associated with higher risk, unknown or suspected poor functional capacity, an objective evaluation of functional capacity should be made if the results are likely to change the peri-operative management.7,10
Frailty, characterised by an increased vulnerability to adverse health outcomes and commonly associated with ageing, is now increasingly recognised as a significant factor in peri-operative risk assessment. Adjusting risk evaluation based on frailty assessment using a validated screening tool among patients aged 70 years or older in addition to functional capability measures, such as self-reported ability to climb two flights of stairs, carries an ESC/ESA class IIa, level B recommendation.8,28,29
Risk prediction tools
Risk prediction scores may assist in quantification of peri-operative cardiovascular risk. Several risk prediction indices have been proposed based on multivariate analyses of observational data.8 These risk calculators combine a mix of clinical factors and surgery-related factors and have less emphasis on frailty and detailed functional assessment. They also do not integrate biomarkers.
The Revised Cardiac Risk Index (RCRI), a modified version of the original Goldman Risk Index, has moderate accuracy in predicting risk of acute coronary syndromes and cardiovascular mortality, but has not been calibrated to other cardiovascular endpoints.14,23 A systematic review of 24 studies with more than 790 000 patients concluded that the RCRI is moderately effective in distinguishing between patients at low versus high risk of cardiac events after non-cardiac surgery. However, the RCRI was found to be inadequate in predicting cardiac events following vascular surgery and in predicting overall patient mortality.30
The National Surgical Quality Improvement Program (NSQIP) risk calculator from the American College of Surgeons (ACS) was developed based on data from over 1 400 000 patients and has been well validated.31,32 It is designed to predict the likelihood of a negative outcome, such as a complication or death, following surgery, and provides information specific to surgery type. Some comparative studies suggest the ACS NSQIP risk calculator has greater peri-operative discriminatory ability in predicting adverse outcomes than previous indices, others suggest it may be less accurate in some surgical types, such as robot-assisted major surgery and colorectal surgery.29,31,33,34
The American Society of Anesthesiologists (ASA) Physical Status Classification System is a broader subjective assessment of overall physical status in the pre-operative setting.35 In one study of 6301 patients, the risk of cardiac complications and mortality for healthy patients (ASA, class I) was 0.1%, and patients with health consistent with ASA class IV had an 18% risk (Box 3).36 A more recently developed risk score is the American University of Beirut (AUSB)-HAS2 Cardiovascular Risk Index, which large studies have suggested has higher accuracy compared with RCRI, but there is less experience with this to date.37,38,39 The most recent ESC/ESA guidelines did not specifically recommend one risk score over another.8
Cardiology consultation and investigations
Current international guidelines recommend against routine specialist cardiac investigations in the work-up of patients undergoing planned non-cardiac surgery.7,10 These infer that low risk patients on clinical risk assessment do not require further investigation. These guidelines also infer that for elevated risk patients, further testing is often for a usual clinical indication and rarely solely for pre-operative work-up, and also that consideration is given to how and whether peri-operative medical, anaesthetic or surgical approaches could be changed.6,40
Transthoracic echocardiography
Although routine pre-operative evaluation of left ventricular function is not recommended by current international guidelines, resting transthoracic echocardiography may be indicated to evaluate valvular function in patients with a newly detected murmur and clinical signs or symptoms of severe valvular disease, including dyspnoea, angina, oedema, recent syncope, or where there may be unexplained dyspnoea or heart failure with worsening clinical status. This is especially so in patients with poor functional capacity before high risk surgery (ESC/ESA, class I level B). Reassessment of left ventricular function in patients with clinically stable heart failure or known moderate to severe valvular disease may be reasonable if echocardiography has not been performed within the past 12 months.6,7,8 Pre-operative findings of left ventricular systolic dysfunction, reduced left ventricular ejection fraction, moderate to severe left ventricular hypertrophy, moderate to severe mitral regurgitation, or aortic stenosis with a mean gradient of 40 mmHg or more have been shown in observational studies to be independently associated with worse peri-operative outcomes, particularly post-operative decompensated heart failure.41,42
Stress testing
Stress exercise ECG, or pharmacological stress testing in patients unable to exercise, has varied recommendations in the current major guidelines. It is not routinely recommended before non-cardiac surgery by Canadian guidelines.16 In contrast, ACC/AHA and ESC/ESA recommend consideration of non-invasive pharmacological stress testing (dobutamine stress echocardiogram or stress myocardial perfusion imaging) in patients who have an elevated clinical risk profile and poor functional capacity (less than four metabolic equivalents of task), if it will change management. Non-invasive stress testing is not recommended by ACC/AHA in patients with a low risk profile, good to excellent exercise tolerance, or those undergoing low risk non-cardiac surgery.7,8 Given formal exercise testing is resource intensive and not easily accessible to all, initial screening with the M-DASI-4Q is a pragmatic approach, consistent with most guidelines, with further objective assessment of functional capacity with formal exercise testing at the discretion of clinicians.27
Biomarker testing
Routine pre-operative biomarker assessment, including high sensitivity cardiac troponin T/I (hs-cTnT/I), B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), has varying recommendations by the major guidelines. Multiple meta-analyses and systematic reviews have demonstrated that pre-operative BNP is associated with short and long term mortality and MACE.43,44,45 One such systematic review and meta-analysis of 2179 patients from 18 studies found that elevated pre-operative BNP (> 92 mg/L) or NT-proBNP (> 300 ng/L) was the strongest independent predictor of death and non-fatal myocardial infarction at 30 days (odds ratio [OR], 3.7; 95% CI, 2.2–6.2; P < 0.001) and at 180 days or more (OR, 2.2; 95% CI, 1.9–2.7; P < 0.001) after surgery.45 Yet, there is no consensus on thresholds at which increased risk is conferred and on how they add value to existing risk prediction strategies.16,46,47 In a prospective observational study of 979 patients, elevated pre-operative hsTnT was the strongest independent predictor for the combined endpoint of in-hospital mortality, myocardial infarction, cardiac arrest, cardiopulmonary resuscitation, and acute decompensated heart failure (hazard ratio, 2.6; 95% CI, 1.3–5.3; P = 0.01).48 Similarly, a systematic review of 19 studies with 13 386 patients undergoing non-cardiac surgery found that pre-operative cardiac troponin is a predictor of short (OR, 4.3; 95% CI, 2.9–6.5; P < 0.001) and long term (OR, 4.2; 95% CI, 1.0–17.3; P = 0.05) MACE and/or all-cause mortality.49 The CCS guidelines recommend routine measurement of BNP or NT-proBNP before non-cardiac surgery in patients aged over 65 years, patients aged 45–64 years with significant CVD, or RCRI score of 1 or over, although they do not advise pre-operative troponin measurement.6 ACC/AHA guidelines do not recommend routine pre-operative BNP measurement and suggest functional capacity as a discriminator for the need for biomarker testing.7 ESC/ESA guidelines suggest a judicious approach with measurement of pre-operative hs-cTnT/I before intermediate and high risk non-cardiac surgery in patients with known CVD; cardiovascular risk factors, including age 65 years or older; or signs or symptoms suggestive of CVD.8,50
Coronary angiography and revascularisation
Coronary computed tomography angiography findings have been shown to correlate with risk of post-operative MACE.40,51 However, there is no evidence from randomised control trials (RCTs) that routine prophylactic revascularisation to prevent ischaemia at the time of surgery improves outcomes in asymptomatic patients or in those with stable coronary artery disease. Hence, routine coronary evaluation with invasive coronary angiography or coronary computed tomography angiography is not generally recommended, and pre-operative coronary revascularisation in this setting to reduce perioperative cardiac events is also not recommended.6,7,8
However, in patients with unstable angina, an individual risk–benefit assessment is necessary to determine the value of coronary revascularisation before urgent or semi-urgent non-cardiac surgery.6 Pre-operative percutaneous coronary intervention (PCI) before non-cardiac surgery may be considered in patients with refractory symptoms, high degree of myocardial ischaemia, or significant angiographic findings, such as left main coronary artery disease, who are unsuitable for bypass surgery. However, these recommendations are less clear-cut and require discussion between the cardiologist and the surgeon.8
In patients requiring PCI before non-cardiac surgery, there is an ESC/ESA class I level A recommendation for new generation drug-eluting stent over bare metal stent and balloon angioplasty. This is based on data from a subgroup analysis of 2432 patients from the LEADERS FREE trial, a randomised, double-blind control study, which demonstrated that new generation drug-eluting stents have superior safety outcomes compared with bare metal stents in patients undergoing early non-cardiac surgery (within three months) following PCI.7,8,52 After elective PCI, it is recommended to delay time-sensitive non-cardiac surgery for a minimum of one month while dual antiplatelet therapy (DAPT; aspirin + P2Y12 inhibitor) is given (ESC/ESA, class I level C).8
Among patients with recent acute coronary syndrome and/or PCI, the risk of peri-operative MACE is higher, and timing of non-cardiac surgery is a balance between the risks of delaying surgery and the risks of ischaemia and stent thrombosis. Guidelines recommend elective non-cardiac surgery be delayed for at least six months after elective PCI and 12 months after acute coronary syndrome, regardless of the revascularisation strategy (ESC/ESA, class I level A).7
In the event of acute coronary syndrome in a patient awaiting a time-sensitive non-cardiac procedure, if surgery can be safely postponed for at least three months, it is recommended that this is done (ESC/ESA, class I level A). In the scenario where critical non-cardiac surgery is needed simultaneously with an acute coronary event requiring revascularisation, a minimalist strategy involving plain balloon angioplasty and delayed stenting may be deemed appropriate, but it should follow a case-by-case approach (ESC/ESA, class IIa level C).8 Our recommendation for coronary revascularisation and timing of non-cardiac surgery is in keeping with the updated 2022 ESC/ESA guidelines.
Preventive management to reduce peri-operative cardiovascular events
Peri-operative cardiovascular preventive management can best be considered a balance between risks and one that is generally optimised through a discussion of these risks in a multidisciplinary team including the patient (Box 4). When unplanned urgent or emergency surgery is required, there is obviously less time to inform these decisions or implement preventive management.
Antiplatelet therapy
A common challenge for clinical decision making is how to manage antiplatelet therapy peri-operatively. A recent systematic review identified 38 relevant studies and attempted to address timing of stopping antiplatelets, continuation versus stopping in patients with stents, and use of bridging therapies.53 With respect to stopping antiplatelets or not, the largest body of evidence was for the question of continuing aspirin versus placebo, with three RCTS and two cohort studies including 28 062 patients, of which the most recent RCT was the POISE-2 trial published in 2014.54 Aspirin continuation was associated with an increased risk of major bleeding (relative risk [RR], 1.31; 95% CI, 1.15–1.50) but did lower the risk of major thromboembolism (stroke, transient ischaemic attack, myocardial infarction, pulmonary embolism, venous thromboembolism, vascular death) (RR, 0.75; 95% CI, 0.59–0.95).9,53,55,56,57,58 Among a small number of studies that examined the benefits of stopping aspirin more than seven days before surgery compared with stopping seven days or less before surgery, there was no difference in risk of bleeding or major thromboembolism.55,59 No well powered trials directly examined continuation of aspirin versus stopping in patients with stents. One large scale secondary analysis of 28 029 patients who had undergone non-cardiac operations within 24 months of stent implantation demonstrated a strong correlation of timing of surgery with MACE (MACE rate 11.6% if < 6 weeks since stent implantation, 6.4% if 6 weeks to less than 6 months, 4.2% if 6–12 months, and 3.5% if >12–24 months).60 The three factors most strongly associated with additional risk increases in patients with stents were non-elective operation, history of recent myocardial infarction less than 6 months, and prior RCRI score greater than 2.60 The RCT evidence on the continuation of long term antiplatelet therapy in peri-operative patients is very limited, and thus recommendations suggest a nuanced approach guided by an accurate assessment of the competing risks of peri-operative thrombotic versus bleeding events.61 The nature of surgery is also key in decision making, including taking into consideration the bleeding risk.
In patients without a history of ischaemic heart disease, or who have not had previous coronary stenting, initiation or continuation of aspirin is not beneficial in those undergoing elective non-cardiac, non-carotid surgery, based on the results from the POISE-2 study.6,7
Antiplatelet therapy in chronic coronary syndrome
It is strongly recommended that antiplatelet management in patients with recent PCI be discussed between the surgeon, the anaesthetist and the cardiologist.7,8 DAPT should be continued for a minimum of one month, and ideally six months, after elective PCI. In patients with a history of PCI, aspirin is recommended to continue peri-operatively. The caveat to this is patients undergoing high bleeding risk surgery, such as intracranial, spinal neurosurgery or vitreoretinal eye surgery, in which case it is recommended to interrupt aspirin for at least seven days before the operation, given the bleeding consequences may be much more significant.8,62
Antiplatelet therapy in acute coronary syndrome
In general, patients with acute coronary syndrome require DAPT for 12 months, unless there is a high risk of bleeding or a need to co-administer oral anticoagulant therapy. Interruption of DAPT is associated with a significant risk of acute stent thrombosis, and such a decision should only be made after careful consideration of risks and benefits and discussion with the treating cardiologist. When requiring time-sensitive surgery following acute coronary syndrome, a minimum duration of three months for DAPT should be considered and aspirin should be continued while P2Y12 inhibition is interrupted, unless planned for high bleeding risk surgery.8,62,63
If interruption of P2Y12 inhibition is indicated, it is recommended to withhold ticagrelor for three to five days, clopidogrel for five days and prasugrel for seven days before non-cardiac surgery (ESC/ESA, class I level B).8,50 If antiplatelet therapy has been withheld before surgery, it is recommended to recommence therapy as soon as possible, ideally within 48 hours after the procedure. However, this requires discussion of risks between the surgeon and the cardiologist (ESC/ESA and ACC/AHA, class I level C).7,8,50
Statin therapy
Peri-operative statin use has been shown in observational data to have a beneficial effect on the 30-day rate of MACE and mortality, as well as on long term mortality and MACE.64 In one large retrospective analysis of 204 885 patients undergoing non-cardiac surgery, patients who were prescribed lipid-lowering therapy had reduced in-hospital mortality compared with patients who were not (2.1% v 3.1% respectively; adjusted OR, 0.62; 95% CI, 0.58–0.67).65 Statin therapy is also associated with a decreased risk of complications after endovascular repair of abdominal aortic aneurysms and reduced risk of stroke after carotid stenting.66,67
In patients already taking statins, peri-operative continuation is strongly recommended (ESC/ESA, class I level B).8 Peri-operative statin withdrawal more than four days after aortic surgery is associated with a threefold increased risk of post-operative myocardial ischaemia.68
Regarding pre-operative commencement of statin therapy in patients not previously taking them, the results from mainly small RCTs and meta-analyses have been inconsistent.69,70 Routine peri-operative initiation of statin therapy is therefore not recommended, unless patients have coronary artery disease or raised cardiovascular risk and would hence be indicated for statins for secondary or high risk primary prevention.8 In addition, the ACC/AHA and ESC/ESA guidelines suggest patients planned for vascular surgery should be initiated on statin therapy at least two weeks before intervention and continued for at least one month following surgery, although there is only moderate evidence to support this (ESC/ESA, class IIa, level B).7,8 Statins with a long half-life, such as atorvastatin, are preferred in the peri-operative period when there may be limited oral intake.8 In summary, long term statin therapy should be continued peri-operatively, and it is reasonable to commence lipid-lowering therapy in patients with high cardiovascular risk and all patients undergoing vascular surgery.
ß-Blockers
There is some theoretical support for the use of ß-blockers in the peri-operative setting because they reduce mismatch in myocardial oxygen supply and demand. Despite observational studies suggesting the use of ß-blockers may improve outcomes in high risk patients, RCTs have found initiating ß-blockers may reduce MACE, but increase total mortality, stroke and clinically significant hypotension or bradycardia.71 Therefore, routine initiation of ß-blockers peri-operatively is not recommended.8 However, most guidelines advise continuation of ß-blockers in patients taking them chronically (ESC/ESA, class I level B), as peri-operative withdrawal is associated with an increased risk of mortality.7,72 But if patients are hypotensive, it is reasonable to reduce or withhold the dose.6,7,8 In patients with a history of ischaemic heart disease, cerebrovascular disease, renal insufficiency or diabetes mellitus, or in those planned for high risk surgical procedures, including vascular surgery, the ESC/ESA guidelines suggest initiation of ß-blockers at least one week before surgery.8,73,74 The ESC/ESA guidelines also give preference to low dose atenolol or bisoprolol, with dose titration to achieve a resting heart rate between 60 and 70 beats per minute with systolic blood pressure greater than 100 mmHg.8 The ACC/AHA and CCS guidelines are similar to the ESC/ESA guidelines.6,7
Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers
There is conflicting advice regarding the management of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) therapy in the peri-operative period. A pooled analysis of three RCTs with a total of 188 patients found that peri-operative continuation of ACE inhibitors or ARBs correlates with increased rates of intra-operative hypotension (pooled RR, 2.53; 95% CI, 1.08–5.93).6,75,76,77 In addition, two large multicentre RCTs have demonstrated that clinically significant hypotension in patients undergoing non-cardiac surgery is independently associated with an elevated risk of death, myocardial infarction, and stroke.71,78 Furthermore, an observational study including 4802 patients undergoing non-cardiac surgery demonstrated that interruption of ACE inhibitors or ARBs before the operation correlated with a reduced risk of clinically significant hypotension (adjusted RR, 0.80; 95% CI, 0.73–0.88) and the composite endpoint of MINS, stroke and mortality at 30 days (adjusted RR, 0.82; 95% CI, 0.70–0.96).79 The CCS guidelines reflect these findings in their support for withholding ACE inhibitors or ARBs for 24 hours before non-cardiac surgery, particularly if there is concomitant ß-blocker use.6 The ACC/AHA guidelines recommend continuation of ACE inhibitors or ARBs peri-operatively, and, if withheld, to recommence them as soon as clinically possible after the operation.7 The ESC/ESA guidelines suggest withholding ACE inhibitors or ARBs on the day of surgery only if they have been prescribed in patients who do not have ejection fraction reduced heart failure.8
Intra-operative management
Events during surgery significantly increase the risk of post-operative cardiac complications. A meta-analysis of 11 studies identified that surgical factors, such as intra-operative bleeding requiring blood transfusion, or prolonged operative time greater than 3.8 hours, as well as haemodynamic factors, including intra-operative tachycardia, hypertension or hypotension, were found to be associated with increased risk of peri-operative cardiac events.80 Intra-operative hypotension is associated with an increased risk of cardiac complications (OR, 2.69; 95% CI, 1.31–5.55; systematic review, n = 130 862). However, there is no standard definition of intra-operative hypotension. A recently reported trial compared hypotension-avoidance (where blood pressure medicines were managed and intra-operative mean arterial pressure = 80 mmHg was targeted) with hypertension-avoidance strategies (where blood pressure medicines were given and intra-operative mean arterial pressure = 60 mmHg was targeted). This study found no difference on major vascular complications at 30 days after the operation.81 The risk of adverse outcomes is related to the degree of hypotension and duration, with the threshold of potential harm beginning at mean arterial pressure below 80 mmHg and duration of more than ten minutes.82 We recommend discussion and shared decision making with the surgeon and anaesthetist to determine the most appropriate procedural and anaesthetic approach. Furthermore, in higher risk patients requiring urgent surgery, more invasive haemodynamic monitoring, intra-operative management to minimise rapid changes in volume status, and maintaining blood pressure and heart rate within a normal range could assist.8,83 In addition, a recent trial, the Perioperative Ischemic Evaluation-3 (POISE-3) trial, suggests the consideration of tranexamic acid intra-operatively. POISE-3 found use of tranexamic acid intra-operatively in major non-cardiac surgery reduced major bleeding (9.1% v 11.7%; absolute difference, -2.6%; 95% CI, -3.8 to -1.4). The non-inferiority of the primary composite cardiovascular safety outcome was not met, although the difference was small (hazard ratio, 1.02; 95% CI, 0.92–1.14).84
Lifestyle management, cardiovascular risk factors, and prehabilitation
Pre-operative exercise intervention, or prehabilitation, has been demonstrated to increase pre-operative functional capacity and to aid recovery following surgery.85 Prehabilitation, in broad terms, involves exercise, nutrition and psychological counselling. Small RCTs have demonstrated improvements in the six-minute walk test, anxiety, depression, and quality of life after major non-cardiac surgery but have been insufficiently powered to examine the impact on peri-operative MACE.86,87 This evidence has influenced guidelines recommending consideration of referral to prehabilitation. However, current access to these types of prehabilitation programs is currently limited.
It is conceivable that prehabilitation could reduce peri-operative MACE, larger scale trials are required, but it makes sense to use the opportunity that prehabilitation offers to optimise peri-operative management and encourage lifestyle risk factor management in the multidisciplinary setting. Pre-operative smoking cessation is recommended — smokers have worse outcomes at one year after surgery and early pre-operative smoking cessation is associated with a lower likelihood of smoking resumption after the operation. In addition, optimisation of cardiovascular risk factors, including blood pressure, dyslipidaemia and diabetes, is strongly recommended before elective non-cardiac surgery.8,88
Post-operative monitoring and management
Peri-operative myocardial infarction, in contrast to non-operative myocardial infarction, is mostly asymptomatic. In the POISE trial, only 34.7% of patients with myocardial infarction reported chest pain.89 In another peri-operative trial, chest pain was present in only 6% of patients with a myocardial infarction and was associated with a 30-day and one-year mortality of 9% and 22% respectively.2 Therefore, routine post-operative clinical assessment facilitates the opportunity for early detection of cardiac complications. Severe post-operative pain increases sympathetic drive and has been shown to be significantly associated with myocardial injury. Avoidance of acute post-operative pain is recommended (ESC/ESA, class I level B).8,90
Electrocardiogram, biomarkers and telemetry
It is common standard of care that high risk patients are monitored closely for a period after their operation, often with a combination of frequent vital sign monitoring, telemetry, ECGs and biomarkers. However, there is a dearth of evidence to support the utility of such monitoring and, thus, guidelines interpret existing evidence differently and recommend differing practices. For example, the ACC/AHA guidelines recommend only performing post-operative ECGs if there are signs or symptoms suggestive of myocardial ischaemia, myocardial infarction, or arrhythmia.7 However, the CCS guidelines recommended routine post-operative ECGs and daily troponin monitoring for 48–72 hours in patients at elevated risk, defined by elevated BNP or proBNP before surgery, RCRI score of 1 or more, age 45–64 years with significant CVD, or age 65 years or older.6 Given their recommendations for daily troponin, Canadian guidelines do not recommend routine telemetry monitoring, as they propose telemetry adds no further benefit.91 Post-operative elevated troponin T has been shown to be a strong predictor of 30-day mortality, but the ACC/AHA guidelines only recommend measuring post-operative troponin if there is clinical evidence of myocardial ischaemia or infarction.5,7 We suggest a tailored approach of increased monitoring for high risk patients using a combination of the above in the immediate post-operative period. Further studies are required to better establish the most efficacious and cost-effective means of monitoring these patients.
Post-operative management
Management of patients with peri-operative cardiovascular complications should be tailored towards the presumed underlying mechanism. Patients who develop MINS, identified through troponin monitoring, are at increased risk for recurrent MACE and mortality in the one to two years after surgery.92 These patients should have ongoing follow-up with a cardiologist to monitor their progress, intensify their cardiovascular medications and arrange further cardiac evaluation as appropriate.6 Notably, in a large observational study of 667 patients with elevated serum troponin after vascular surgery, patients with MINS who were prescribed intensified medical therapy had a one-year survival free from a major cardiac event rate similar to surgical patients without MINS.93 Commencement of aspirin and statin after MINS has been demonstrated to have a significant reduction in 30-day mortality.89,93 In addition, evidence from an international RCT demonstrated that, in patients who had MINS after non-cardiac surgery, the commencement of dabigatran 110 mg twice a day reduced the risk of major vascular complications, without significantly increasing major bleeding.94
Conclusions
The literature to inform peri-operative cardiovascular risk management has grown, but there are still many unanswered questions (Box 5). Cardiac risk assessment should be communicated to the patient to allow enhanced shared decision making in the peri-operative setting.6,8 For patients who are assessed as being at very high risk of peri-operative morbidity or mortality, this may influence the decisions regarding delaying surgery to allow optimisation or prehabilitation, cancelling or proceeding with surgery, or taking a more conservative approach. In patients at high risk of proceeding to surgery, there is limited evidence that any specific therapy or interventions can mitigate peri-operative risk, but optimal guideline-based management of cardiovascular conditions and an individualised approach are recommended. Likewise, for patients who experience peri-operative cardiovascular complications, the evidence on which to base treatment is limited and strategies need to consider individual circumstances.
Box 1 – Factors predictive of increased peri-operative cardiac risk
|
Predictive factors: patient characteristics |
Predictive surgical factors |
||||||||||||||
|
|
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Modified four-question Duke Activity Status Index (M-DASI-4Q)
- Are you able to climb a flight of stairs or walk up a hill?
- Are you able to do heavy work around the house (lifting and moving heavy furniture)?
- Are you able to do yard work (raking leaves or pushing a power mower)?
- Are you able to participate in strenuous sports (swimming, singles tennis, football, basketball or skiing)?
Box 3 – Risk assessment scores for patients undergoing surgery
|
Revised Cardiac Risk Index (RCRI; 1999)*14 |
National Surgical Quality Improvement Program (NSQIP) — Surgical Risk Calculator (2013)31 |
American Society of Anesthesiologists (ASA) risk score36 |
|||||||||||||
|
|
|||||||||||||||
|
|
|
|||||||||||||
|
|
|||||||||||||||
|
* Score 1 point for each risk of complications: 0 = 0.4%; 1 = 0.9%; 2 = 7%; = 3 = 11%. |
|||||||||||||||
Box 4 – Shared decision to balance risk with surgical urgency and optimise peri-operative management to reduce cardiovascular risk
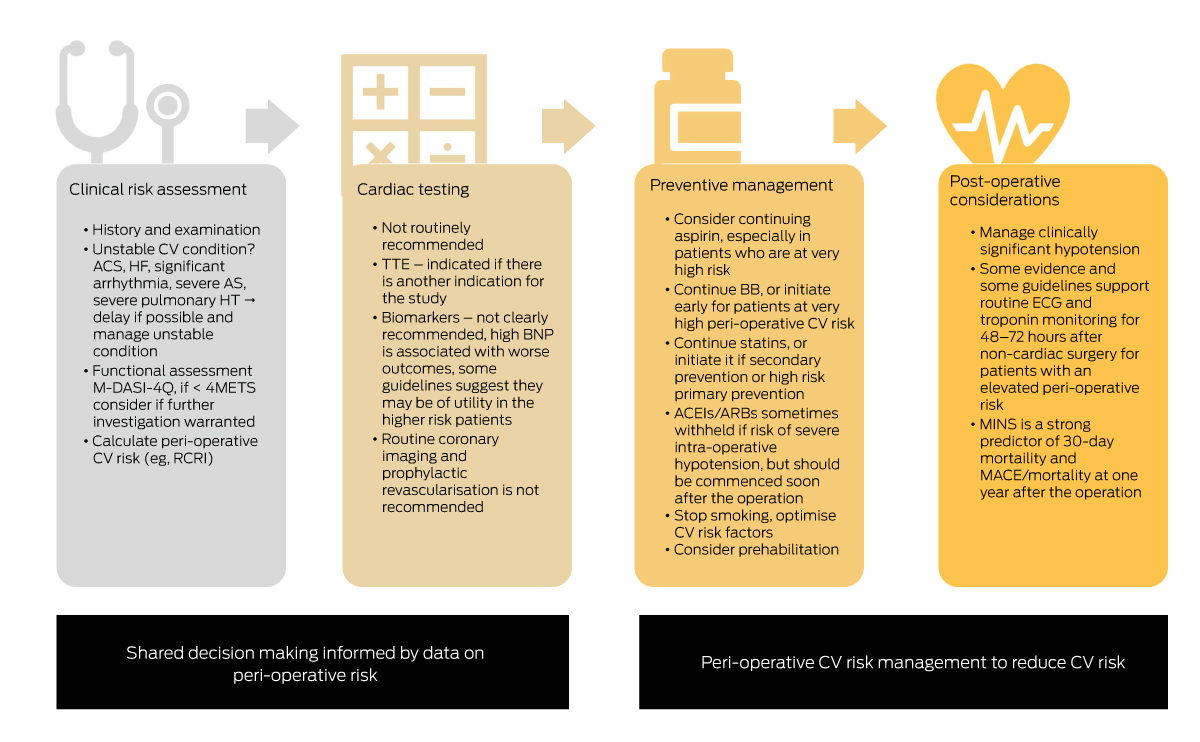
ACEIs = angiotensin-converting enzyme inhibitors; ACS = acute coronary syndrome; ARBs = angiotensin-receptor blockers; AS = aortic stenosis; BB = ß-blocker; BNP = B-type natriuretic peptide; CV = cardiovascular; ECG = electrocardiogram; HF = heart failure; HT = hypertension; MACE = major adverse cardiovascular events; M-DASI-4Q = Modified four-question Duke Activity Status Index; METS = metabolic equivalents of task; MINS = myocardial injury after non-cardiac surgery; RCRI = Revised Cardiac Risk Index; TTE = transthoracic echocardiogram.
Box 5 – Gaps in the evidence and trials underway to address them
|
|
|||||||||||||||
|
Diagnostic tests and prediction scores for peri-operative risk evaluation |
|||||||||||||||
|
|||||||||||||||
|
Peri-operative therapeutic medications to improve post-operative outcomes |
|||||||||||||||
|
|||||||||||||||
|
Peri-operative cardiovascular medication management |
|||||||||||||||
|
|||||||||||||||
|
Intra-operative blood pressure management |
|||||||||||||||
|
|||||||||||||||
|
Utility of prehabilitation before non-cardiac surgery* |
|||||||||||||||
|
|||||||||||||||
|
Management of peri-operative cardiovascular events |
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
* There are no current large clinical trials to examine the impact of prehabilitation on major adverse cardiovascular events for patients undergoing major non-cardiac surgery. |
|||||||||||||||
Provenance: Commissioned; externally peer reviewed.
- Shehane Mahendran1
- Aravinda Thiagalingam2
- Graham Hillis3
- Richard Halliwell2
- Henry CC Pleass4
- Clara K Chow2,5
- 1 Westmead Applied Research Centre, University of Sydney, Sydney, NSW
- 2 Westmead Hospital, Sydney, NSW
- 3 Royal Perth Hospital, Perth, WA
- 4 Institute of Academic Surgery, Royal Prince Alfred Hospital, Sydney, NSW
- 5 University of Sydney, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Clara Chow is recipient of a National Health and Medical Research Council Investigator Grant (APP1195326).
- 1. Australian Institute of Health and Welfare: Hospitals at a glance 2017–18 [Cat. No. HSE 232]. AIHW, 2019. https://www.aihw.gov.au/reports/hospitals/hospitals‐at‐a‐glance‐2017‐18 (viewed Feb 2023).
- 2. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137: 1221‐1232.
- 3. Smilowitz NR, Gupta N, Ramakrishna H, et al. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2: 181‐187.
- 4. Sazgary L, Puelacher C, Lurati Buse G, et al. Incidence of major adverse cardiac events following non‐cardiac surgery. Eur Heart J Acute Cardiovasc Care 2020; 10: 550‐558.
- 5. Writing Committee for the VISION Study Investigators; Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high‐sensitivity troponin levels with myocardial injury and 30‐day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317: 1642‐1651.
- 6. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33: 17‐32.
- 7. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64: e77‐e137.
- 8. Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non‐cardiac surgery: Developed by the task force for cardiovascular assessment and management of patients undergoing non‐cardiac surgery of the European Society of Cardiology (ESC) Endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC). Eur Heart J 2022; 43: 3826‐3924.
- 9. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370: 1494‐1503.
- 10. Smilowitz NR, Berger JS. Perioperative cardiovascular risk assessment and management for noncardiac surgery: a review. JAMA 2020; 324: 279‐290.
- 11. Mullen MG, Michaels AD, Mehaffey JH, et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining “quality” and reporting outcomes for urgent surgery. JAMA Surg 2017; 152: 768‐774.
- 12. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373: 2258‐2269.
- 13. Van Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation 2011; 124: 289‐296.
- 14. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043‐1049.
- 15. Kumar R, McKinney WP, Raj G, et al. Adverse cardiac events after surgery: assessing risk in a veteran population. J Gen Intern Med 2001; 16: 507‐518.
- 16. Soucy‐Giguere M, Duceppe E, Shi S, et al. Correlation between preoperative electrocardiogram findings and NT‐ProBNP and postoperative cardiac events after noncardiac surgery. Canadian Journal of Cardiology 2021; 37 (Suppl): S105‐S106.
- 17. Studzińska D, Polok K, Rewerska B, et al. Prognostic value of preoperative electrocardiography in predicting myocardial injury after vascular surgery. Kardiol Pol 2022; doi: https://doi.org/10.33963/KP.a2022.0085 [Epub ahead of print].
- 18. Sapra R, Hallqvist L, Schlegel TT, et al. Predicting peri‐operative troponin elevation by advanced electrocardiography. J Electrocardiol 2021; 68: 1‐5.
- 19. Reilly DF, McNeely MJ, Doerner D, et al. Self‐reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999; 159: 2185‐2192.
- 20. Levett D, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth 2018; 120: 484‐500.
- 21. Lurati Buse GAL, Puelacher C, Gualandro DM, et al. Association between self‐reported functional capacity and major adverse cardiac events in patients at elevated risk undergoing noncardiac surgery: a prospective diagnostic cohort study. Br J Anaesth 2021; 126: 102‐110.
- 22. Marsman M, van Waes JAR, Grobben RB, et al. Added value of subjective assessed functional capacity before non‐cardiac surgery in predicting postoperative myocardial injury. Eur J Prev Cardiol 2020; 28: 262‐269.
- 23. Wotton R, Marshall A, Kerr A, et al. Does the revised cardiac risk index predict cardiac complications following elective lung resection? J Cardiothorac Surg 2013; 8: 220.
- 24. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self‐administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989; 64: 651‐654.
- 25. Wijeysundera DN, Beattie WS, Hillis GS, et al. Integration of the Duke Activity Status Index into preoperative risk evaluation: a multicentre prospective cohort study. Br J Anaesth 2020; 124: 261‐270.
- 26. Davies SJ, Minto G. Occam's razor at the sharp end: simplified preoperative risk assessment. Br J Anaesth 2021; 126: 27‐30.
- 27. Riedel B, Li MH, Lee CHA, et al. A simplified (modified) Duke Activity Status Index (M‐DASI) to characterise functional capacity: a secondary analysis of the Measurement of Exercise Tolerance before Surgery (METS) study. Br J Anaesth 2021; 126: 181‐190.
- 28. Furukawa H. Current clinical implications of frailty and sarcopenia in vascular surgery: a comprehensive review of the literature and consideration of perioperative management. Ann Vasc Dis 2022; 15: 165‐174.
- 29. Narain AS, Kitto AZ, Braun B, et al. Does the ACS NSQIP surgical risk calculator accurately predict complications rates after anterior lumbar interbody fusion procedures? Spine (Phila Pa 1976) 2021; 46: E655‐E662.
- 30. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med 2010; 152: 26‐35.
- 31. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217: 833‐842.
- 32. Yap MKC, Ang KF, Gonzales‐Porciuncula LA, Esposo E. Validation of the American College of Surgeons Risk Calculator for preoperative risk stratification. Heart Asia 2018; 10: e010993.
- 33. Lone Z, Hall S, Terakawa T, et al. Accuracy of American College of Surgeons National Surgical Quality Improvement Program Universal Surgical Risk Calculator in Predicting Complications Following Robot‐Assisted Radical Cystectomy at a National Comprehensive Cancer Center. J Endourol 2019; 33: 383‐388.
- 34. van der Hulst HC, Dekker JWT, Bastiaannet E, et al. Validation of the ACS NSQIP surgical risk calculator in older patients with colorectal cancer undergoing elective surgery. J Geriatr Oncol 2022; 13: 788‐795.
- 35. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 2011;55: 111‐115.
- 36. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 1996; 77: 217‐222.
- 37. Dakik HA, Chehab O, Eldirani M, et al. A new index for pre‐operative cardiovascular evaluation. J Am Coll Cardiol 2019; 73: 3067‐3078.
- 38. Dakik HA, Eldirani M, Kaspar C, et al. Prospective validation of the AUB‐HAS2 cardiovascular risk index. Eur Heart J Qual Care Clin Outcomes 2022; 8: 96‐97.
- 39. Dakik HA, Sbaity E, Msheik A, et al. AUB‐HAS2 Cardiovascular Risk Index: performance in surgical subpopulations and comparison to the Revised Cardiac Risk Index. J Am Heart Assoc 2020; 9: e016228.
- 40. Koshy AN, Ha FJ, Gow PJ, et al. Computed tomographic coronary angiography in risk stratification prior to non‐cardiac surgery: a systematic review and meta‐analysis. Heart 2019; 105: 1335‐1342.
- 41. Rohde LE, Polanczyk CA, Goldman L, et al. Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgery. Am J Cardiol 2001; 87: 505‐509.
- 42. Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112: 1316‐1324.
- 43. Karthikeyan G, Moncur RA, Levine O, et al. Is a pre‐operative brain natriuretic peptide or N‐terminal pro‐B‐type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta‐analysis of observational studies. J Am Coll Cardiol 2009; 54: 1599‐1606.
- 44. Rodseth RN, Padayachee L, Biccard BM. A meta‐analysis of the utility of pre‐operative brain natriuretic peptide in predicting early and intermediate‐term mortality and major adverse cardiac events in vascular surgical patients. Anaesthesia 2008; 63: 1226‐1233.
- 45. Rodseth RN, Biccard BM, Le Manach Y, et al. The prognostic value of pre‐operative and post‐operative B‐type natriuretic peptides in patients undergoing noncardiac surgery: B‐type natriuretic peptide and n‐terminal fragment of pro‐B‐type natriuretic peptide: a systematic review and individual patient data meta‐analysis. J Am Coll Cardiol 2014; 63: 170‐180.
- 46. Vernooij LM, van Klei WA, Moons KG, et al. The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all‐cause mortality in patients who undergo noncardiac surgery. Cochrane Database Syst Rev 2021; (12): CD013139.
- 47. Clerico A, Zaninotto M, Aimo A, et al. Evaluation of the cardiovascular risk in patients undergoing major non‐cardiac surgery: role of cardiac‐specific biomarkers. Clin Chem Lab Med 2022; 60: 1525‐1542.
- 48. Weber M, Luchner A, Seeberger M, et al. Incremental value of high‐sensitive troponin T in addition to the revised cardiac index for peri‐operative risk stratification in non‐cardiac surgery. Eur Heart J 2013; 34: 853‐862.
- 49. Humble CAS, Huang S, Jammer I, et al. Prognostic performance of preoperative cardiac troponin and perioperative changes in cardiac troponin for the prediction of major adverse cardiac events and mortality in noncardiac surgery: A systematic review and meta‐analysis. PLoS One 2019; 14: e0215094.
- 50. Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes 2016. Med J Aust 2016; 205: 128‐133. https://www.mja.com.au/journal/2016/205/3/national‐heart‐foundation‐australia‐and‐cardiac‐society‐australia‐and‐new
- 51. Hwang JW, Kim EK, Yang JH, et al. Assessment of perioperative cardiac risk of patients undergoing noncardiac surgery using coronary computed tomographic angiography. Circ Cardiovasc Imaging 2015; 8: e002582.
- 52. Richardt G, Abdelghani M, Allali A, et al. Polymer‐free drug‐coated vs. bare‐metal coronary stents in patients undergoing non‐cardiac surgery: a subgroup analysis of the LEADERS FREE trial. Clin Res Cardiol 2021; 110: 162‐171.
- 53. Shah S, Urtecho M, Firwana M, et al. Perioperative management of antiplatelet therapy: a systematic review and meta‐analysis. Mayo Clin Proc Innov Qual Outcomes 2022; 6: 564‐573.
- 54. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370: 1494‐1503.
- 55. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence — imprecision. J Clin Epidemiol 2011; 64: 1283‐1293.
- 56. Rahman M, Donnangelo LL, Neal D, et al. Effects of perioperative acetyl salicylic acid on clinical outcomes in patients undergoing craniotomy for brain tumor. World Neurosurg 2015; 84: 41‐47.
- 57. Oscarsson A, Gupta A, Fredrikson M, et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. Br J Anaesth 2010; 104: 305‐312.
- 58. O'Brien J, Duncan H, Kirsh G, et al. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355: 1295‐1302.
- 59. Zeng L, Brignardello‐Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol 2021; 137: 163‐175.
- 60. Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310: 1462‐1472.
- 61. Dargham BB, Baskar A, Tejani I, et al. Intravenous antiplatelet therapy bridging in patients undergoing cardiac or non‐cardiac surgery following percutaneous coronary intervention. Cardiovasc Revasc Med 2019; 20: 805‐811.
- 62. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2018; 39: 213‐260.
- 63. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI); Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J 2010; 31: 2501‐2555.
- 64. London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177: 231‐242.
- 65. Lindenauer PK, Pekow P, Wang K, et al. Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery. JAMA 2004; 291: 2092‐2099.
- 66. McNally MM, Agle SC, Parker FM, et al. Preoperative statin therapy is associated with improved outcomes and resource utilization in patients undergoing aortic aneurysm repair. J Vasc Surg 2010; 51: 1390‐1396.
- 67. Verzini F, De Rango P, Parlani G, et al. Effects of statins on early and late results of carotid stenting. J Vasc Surg 2011; 53: 71‐9; discussion 9.
- 68. Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg 2007; 104: 1326‐1333.
- 69. Putzu A, Capelli B, Belletti A, et al. Perioperative statin therapy in cardiac surgery: a meta‐analysis of randomized controlled trials. Crit Care 2016; 20: 395.
- 70. Ma B, Sun J, Diao S, Zheng B, Li H. Effects of perioperative statins on patient outcomes after noncardiac surgery: a meta‐analysis. Ann Med 2018; 50: 402‐409.
- 71. POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371: 1839‐1847.
- 72. Shammash JB, Trost JC, Gold JM, et al. Perioperative beta‐blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141: 148‐153.
- 73. London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative β‐blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309: 1704‐1713.
- 74. Angeli F, Verdecchia P, Karthikeyan G, et al. ß‐Blockers reduce mortality in patients undergoing high‐risk non‐cardiac surgery. Am J Cardiovasc Drugs 2010; 10: 247‐259.
- 75. Bertrand M, Godet G, Meersschaert K, et al. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg 2001; 92: 26‐30.
- 76. Coriat P, Richer C, Douraki T, et al. Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction. Anesthesiology 1994; 81: 299‐307.
- 77. Schirmer U, Schürmann W. [Preoperative administration of angiotensin‐converting enzyme inhibitors] [German]. Anaesthesist 2007; 56: 557‐561.
- 78. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370: 1504‐1513.
- 79. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology 2017; 126: 16‐27.
- 80. Skinner DL, Goga S, Rodseth RN, Biccard BM. A meta‐analysis of intraoperative factors associated with postoperative cardiac complications. South Afr J Anaesth Analg 2012; 18: 186‐191.
- 81. Marcucci M, Painter TW, Conen D, et al. Hypotension‐avoidance versus hypertension‐avoidance strategies in noncardiac surgery: an international randomized controlled trial. Ann Intern Med 2023; doi: https://doi.org/10.7326/M22‐3157 [Epub ahead of print].
- 82. Wesselink EM, Kappen TH, Torn HM, et al. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth 2018; 121: 706‐721.
- 83. Marcucci M, Painter TW, Conen D, et al. Rationale and design of the PeriOperative ISchemic Evaluation‐3 (POISE‐3): a randomized controlled trial evaluating tranexamic acid and a strategy to minimize hypotension in noncardiac surgery. Trials 2022; 23: 101.
- 84. Devereaux PJ, Marcucci M, Painter TW, et al; POISE‐3 Investigators. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med 2022; 386:1986‐1997.
- 85. Boukili IE, Flaris AN, Mercier F, et al. Prehabilitation before major abdominal surgery: Evaluation of the impact of a perioperative clinical pathway, a pilot study. Scand J Surg 2022; 111: 14574969221083394.
- 86. Fulop A, Lakatos L, Susztak N, et al. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia 2021; 76: 82‐90.
- 87. Blackwell JEM, Doleman B, Boereboom CL, et al. High‐intensity interval training produces a significant improvement in fitness in less than 31 days before surgery for urological cancer: a randomised control trial. Prostate Cancer Prostatic Dis 2020; 23: 696‐704.
- 88. Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control 2006; 15: 352‐358.
- 89. Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154: 523‐528.
- 90. Turan A, Leung S, Bajracharya GR, et al. Acute postoperative pain is associated with myocardial injury after noncardiac surgery. Anesth Analg 2020; 131: 822‐829.
- 91. Botto F, Alonso‐Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology 2014; 120: 564‐578.
- 92. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319‐1330.
- 93. Foucrier A, Rodseth R, Aissaoui M, et al. The long‐term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119: 1053‐1063.
- 94. Devereaux P, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non‐cardiac surgery (MANAGE): an international, randomised, placebo‐controlled trial. Lancet 2018; 391: 2325‐2334.






Summary