Clinical record
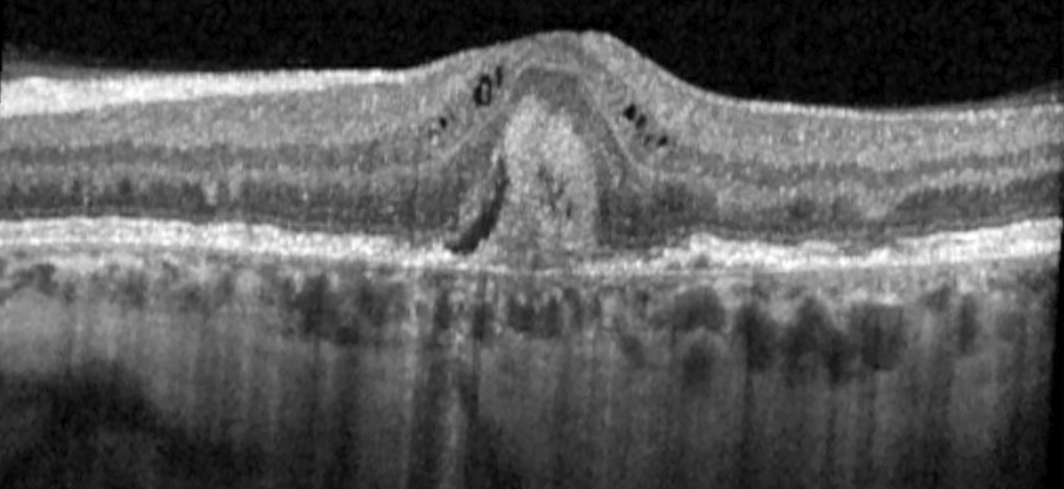
A 55‐year‐old woman in the United States, with a history of interstitial cystitis diagnosed at age 33 years, was referred for pattern macular dystrophy. Her primary complaint was difficulty reading, despite using bifocals. Her medications included hydroxyzine, amitriptyline and pentosan polysulfate (PPS). She had taken 300 mg of PPS daily for 21 years (cumulative dose, 2.3 kg). Visual acuity with correction was 6/7.5 and intraocular pressure was normal in both eyes. Fundus examination revealed bilateral perifoveal pigmentary deposits without haemorrhage or exudate. Spectral domain optical coherence tomography (SD‐OCT; SPECTRALIS HRA + OCT, Heidelberg Engineering), demonstrated irregular retinal pigment epithelium and small pigment epithelial detachments in the maculae of both eyes without cystoid macular oedema. Near‐infrared reflectance imaging and autofluorescence showed hyper‐reflective patches and speckled areas of hypo‐ and hyper‐autofluorescence, respectively, in each eye (Box 1). Fluorescein angiogram demonstrated retinal pigment epithelial staining without leakage. Cessation of PPS and routine monitoring were recommended. Subsequently, the patient developed unilateral choroidal neovascularisation of the left eye (Box 2), which was treated with an intravitreal antivascular endothelial growth factor agent.
Discussion
PPS is prescribed to treat interstitial cystitis (also referred to as “bladder pain syndrome”). Interstitial cystitis is a complex multifactorial disorder with poorly understood pathophysiology. It has been described as pain perceived to be related to the bladder or pelvis accompanied by other urinary symptoms, such as urgency or frequency, not caused by an infection or other identifiable aetiology. The estimated prevalence of interstitial cystitis is 1–3% of women and is less common in men by a factor of five to ten.1 Treatment of interstitial cystitis is challenging, as no single treatment is particularly effective. A multimodal approach to treatment is common and can include behavioural changes, pelvic floor exercises, stress reduction, oral medications (including tricyclic antidepressants, hydroxyzine, and PPS), and/or procedures such as intravesicular therapy with lidocaine.1
PPS maculopathy (PPM) was first identified in an article published in 2018 which reported six patients with a pigmentary maculopathy in the setting of chronic exposure to PPS.2 All patients described prolonged dark adaptation, but the most prominent symptom was difficulty reading (four of six patients) despite an average visual acuity of 6/7.5. Three of the six patients underwent automated static perimetry, which revealed paracentral scotomas in both eyes. On average, the patients had taken PPS for 16 years, with a total cumulative drug dose of 2.2 kg. The shortest duration of PPS use in any patient was 12 years. No patients had choroidal neovascularisation, but one had cystoid macular oedema. In a case series of 70 eyes of 35 patients with PPM, six eyes of nine patients had cystoid macular oedema and one eye had choroidal neovascularisation.3 PPM may progress despite cessation of PPS.4
Fundus findings in PPM include parafoveal pigmentary deposits, macular vitelliform deposits, and paracentral retinal pigment epithelium atrophy. Short wavelength fundus autofluorescence imaging typically demonstrates an irregular pattern of hyper‐ and hypo‐autofluorescence in the macula. Near‐infrared reflectance imaging may reveal irregular hyper‐reflective spots in the macula. SD‐OCT may show nodular excrescences at the level of the retinal pigment epithelium, outer retinal tubulations, and patchy loss of the retinal pigment epithelium. These findings may mimic those of other diseases, such as age‐related macular degeneration.
The Food and Drug Administration (FDA) recently published a review of available evidence in September 2021: “It is important for urogynecologists prescribing pentosan to be aware of this potential association and be vigilant about assessing eye health in pentosan users”.5 In October 2021, the Therapeutic Goods Administration added a safety advisory that PPS is associated with pigmentary maculopathy, though no cases have yet been reported in Australia.6 No evidence‐based guidelines for PPM screening or management exist, but the FDA has advised a baseline retinal examination for all patients within six months of initiating treatment and periodically while continuing treatment. Relevant tests may include colour fundus photos, fundus autofluorescence, and macular SD‐OCT with near‐infrared reflectance imaging. It is unclear whether other macular diseases confer an increased risk of PPM.
PPM is a relatively newly identified macular disease with potentially severe visual consequences, and the understanding of its natural history and risk factors continues to evolve. However, it should be considered as a differential diagnosis of macular pigmentary abnormalities in any patient taking PPS, particularly those with high cumulative drug exposure. Patients taking PPS should be screened regularly to monitor for PPM and should be counselled on this risk. The current unknowns, including risk factors, influence of other pre‐existing retinal or macular diseases, screening frequency and modality, and long term outcome following drug cessation, should be shared with the patient and with the prescribing physician to facilitate informed decision making.
Lessons from practice
- Pentosan polysulfate (PPS) can cause a toxic maculopathy (PPM), leading to visual disturbances, particularly with difficulty reading. This can be irreversible and progressive despite PPS cessation.
- The efficacy of PPS in treating interstitial cystitis is questionable and other treatments may be preferable given the potential complication of PPM.
- Patients taking PPS should be referred to an eye care provider for a baseline examination and routine monitoring, with comprehensive eye exam including dilated biomicroscopy of the retina and macula and multimodal retinal imaging with spectral domain optical coherence tomography, fundus autofluorescence, and near‐infrared reflectance imaging.
- Treat PPM with immediate PPS cessation and consider adjusting therapy for associated complications (eg, intravitreal antivascular endothelial growth factor therapy for choroidal neovascularisation).
Box 1 – Multimodal retinal imaging of the right and left maculae in a patient with retinopathy associated with pentosan polysuflate intake
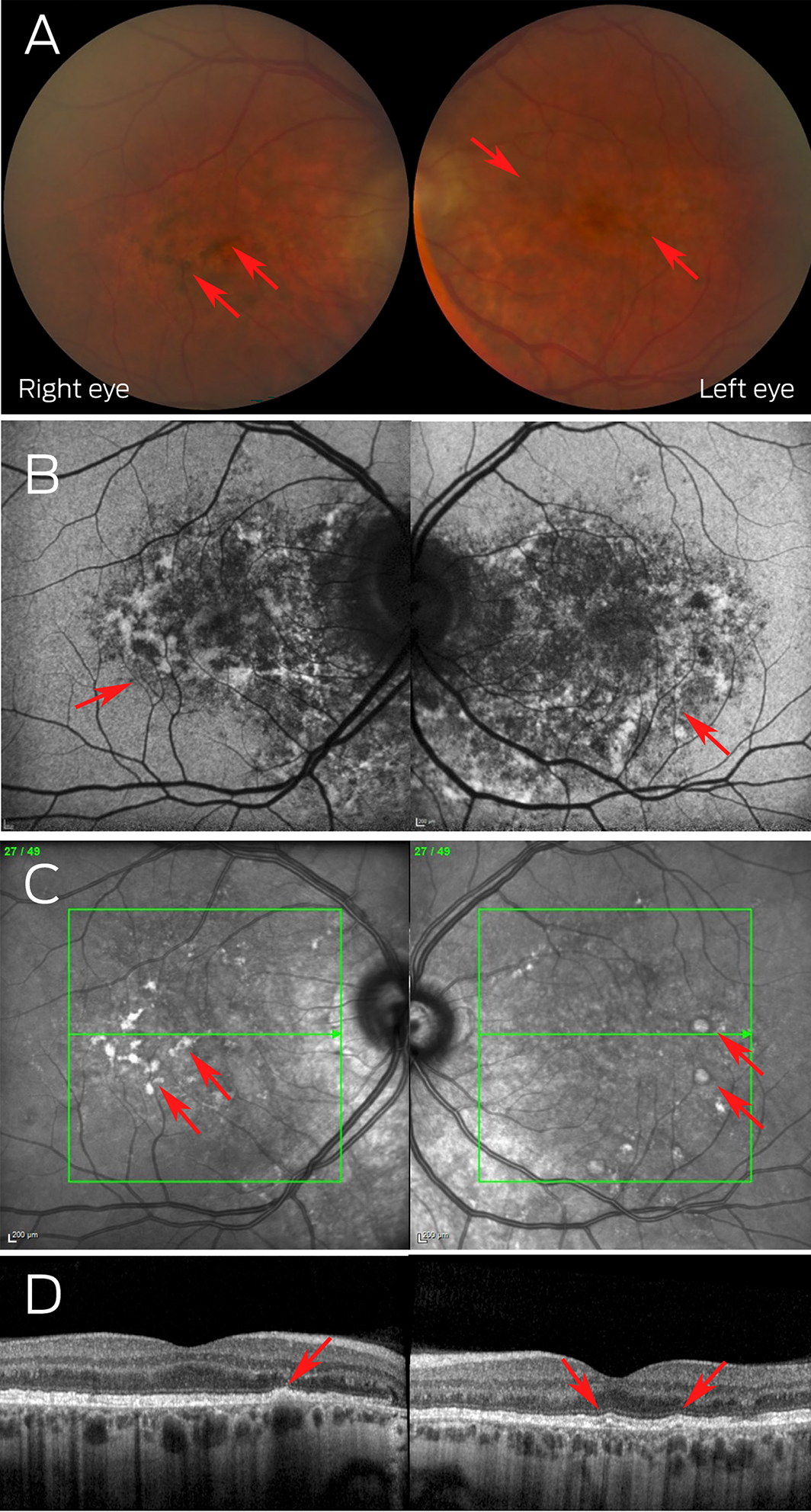
(A) Colour fundus photos showing the perifoveal pigmentary deposits (arrows); (B) autofluorescence fundus photos highlighting macular speckled hyper‐ and hypo‐autofluorescence (arrows); (C) near‐infrared reflectance photos demonstrating hyper‐reflective deposits (arrows); (D) optical coherence tomography highlighting irregularity of the retinal pigment epithelium with small pigment epithelial detachments (arrows).
Provenance: Not commissioned; externally peer reviewed.
- 1. Marcu I, Campian EC, Tu FF. Interstitial cystitis/bladder pain syndrome. Semin Reprod Med 2018; 36: 123‐135.
- 2. Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology 2018; 125: 1793‐1802.
- 3. Hanif AM, Armenti ST, Taylor SC, et al. Phenotypic spectrum of pentosan polysulfate sodium‐associated maculopathy: a multicenter study. JAMA Ophthalmol 2019; 137: 1275‐1282.
- 4. Huckfeldt RM, Vavvas DG. Progressive maculopathy after discontinuation of pentosan polysulfate sodium. Ophthalmic Surg Lasers Imaging Retina 2019; 50: 656‐659.
- 5. Lardieri A, Konkel K, McCulley L, et al. Pentosan associated retinal pigmentary changes: FDA's perspective on an emerging postmarketing safety finding. Int Urogynecol J 2021; 32: 2891‐2897.
- 6. Therapeutic Goods Administration. Pentosan polysulfate sodium (Elmiron). Canberra: Commonwealth of Australia, 2021. https://www.tga.gov.au/news/safety‐alerts/pentosan‐polysulfate‐sodium‐elmiron (viewed Mar 2023).






Open access
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
No relevant disclosures.