Electronic cigarettes (e‐cigarettes, vapes) are devices that aerosolise an “e‐liquid” for inhalation.1,2,3 Devices range from older low power “cigalikes” to refillable pen and larger tank devices, to more recent small high concentration nicotine salt pods and disposable products.2,3,4,5 E‐cigarettes are used by millions of people around the world, particularly by younger people.6,7 In the 2019 Australian National Drug Strategy Household Survey, 26% of 18–24‐year‐old respondents and 10% of those aged 40–49‐years reported having used e‐cigarettes at least once.8
The contemporary evidence on e‐cigarettes, including information about their direct effects on health and indirect effects (eg, impact on smoking behaviour), must be integrated to inform evidence‐based policy and practice. Several major reviews of the health effects of e‐cigarettes have been published,2,3,9,10,11,12 but no contemporary comprehensive systematic reviews or major reports with systematic quality assessment were identified prior to our review for the Australian Department of Health.13 We provide a contemporary overview of the evidence regarding the health effects of nicotine and non‐nicotine e‐cigarettes.
Methods
Our review integrates an umbrella review of evidence from major independent reviews with a top‐up systematic review, following PRISMA guidelines,14 using the same search terms and eligibility criteria. It is based on work commissioned by the Australian Department of Health,13,15 which also provided evidence for the 2021 Royal Australian College of General Practitioners (RACGP) update to smoking cessation guidelines16 and the 2022 statement of the National Health and Medical Research Council (NHMRC) Chief Executive Officer.17
Umbrella review
As our starting point, we used the most recent large scale review, the 2018 United States National Academies of Sciences, Engineering, and Medicine (NASEM) review,3 and we searched for other major reviews in the grey literature to 31 December 2021. Studies included in the NASEM and other major reviews but not already identified by our top‐up review (see below) were evaluated using the same eligibility criteria as the top‐up review, summarised, and integrated into our narrative synthesis. We identified major reviews published after our 2021 search (to the end of November 2022) using rapid search methods (Supporting Information, part 1); these reviews are not included in our results synthesis but are considered in our discussion.
Top‐up review
We searched PubMed, MEDLINE, Scopus, Web of Science, the Cochrane Library, and PsycINFO in July 2020 for relevant publications during July 2017 – July 2020 not included in the NASEM review (details: Supporting Information, part 1; protocol published in PROSPERO: CRD42020200673, 31 August 2020). We included evidence regarding e‐cigarettes and smoking initiation and cessation reported by the current authors in previous systematic reviews in our analysis.15,18,19
Inclusion and exclusion criteria
We included peer‐reviewed original research articles on the health effects of e‐cigarettes. When possible, evidence related to e‐cigarettes delivering tetrahydrocannabinol (THC) was excluded as being outside the scope of the review, as were animal, in vitro, and biomarker studies (Supporting Information, part 1).
Eligibility screening
Article details were imported into EndNote, exported to Covidence (https://www.covidence.org), and duplicates removed. Two authors (two of AY, SB, KB, MM, MN, AD, SC) independently screened all titles, abstracts, and articles according to the eligibility criteria; disagreements were resolved by consensus or by a third author.
Data extraction
One of the authors independently extracted data using a pre‐specified piloted data extraction template; data were checked by a second author. Outcomes were classified by clinical disease endpoints (eg, stroke, myocardial infarction, cancer), subclinical outcomes (eg, coronary artery calcification, lung function), and other health outcomes. Missing data, competing interests, and funding were noted.
Risk of bias assessment
The methodological quality of studies included in the top‐up review was independently assessed by two authors using Joanna Briggs Institute critical appraisal tools (https://jbi.global/critical‐appraisal‐tools). Disagreements were resolved by consensus discussions or by consultation with a third author. Three otherwise eligible studies were excluded because of low quality scores.20,21,22
Integrated data analysis
GRADE assessment
The certainty of the integrated body of evidence for each health outcome derived from the umbrella and top‐up reviews was assessed using the GRADE approach23,24 (Supporting Information, part 2).
Data synthesis
We prioritised study designs most useful for assessing causal effects with respect to the health outcome (as applicable), in the order: randomised controlled trials; prospective cohort studies; case–control studies; and non‐randomised intervention studies. Published meta‐analyses were included when available. For the smoking cessation analyses, we included only randomised controlled trials with biochemically verified cessation outcomes and at least four months of follow‐up. When epidemiological studies were unavailable or not relevant, other potentially informative evidence was examined, including cross‐sectional surveys, case reports and case series (particularly for exposure‐dependent outcomes, such as burns and injuries), and findings from surveillance systems.
We separately summarised the study characteristics and main findings for the umbrella and top‐up reviews, and then integrated them in a narrative synthesis, drawing conclusions on the certainty of the evidence (NASEM framework), by exposure groups and comparators. We conducted meta‐analyses when possible (Supporting Information, part 2).
Results
We identified 6558 potentially eligible publications for the top‐up review. After screening, 227 were eligible for our analysis. A further ten items were identified by forward and backward citation searching, and one was identified in the grey literature. Eighty‐six publications were excluded because the study designs precluded drawing causal inferences about links between e‐cigarette use and health outcomes. A further 37 relevant publications were identified in two earlier reviews of smoking uptake and cessation by the authors.15,18 In total, 189 studies met our selection criteria for the top‐up review (Box 1).
Eight relevant major independent reviews were identified: those prepared for Public Health England (2018, 2020, 2021),11,25,26 the Commonwealth Scientific and Industrial Research Organisation (Australia, 2018),9 NASEM (United States, 2018),3 the Irish Health Research Board (2020),10 the European Union Scientific Committee on Health, Environmental, and Emerging Risks (2021),2 and the United States Preventive Services Task Force (2021).12 A total of 112 relevant studies were identified in the NASEM review, as well as 99 additional studies cited by other reviews and not captured in the top‐up review.
A total of 400 publications were therefore included in the final evidence synthesis (Supporting Information, parts 3 and 4; Box 2).13 Information on nicotine content was generally not provided; however, as almost all e‐cigarette products sold probably contain nicotine,32 we assumed that reported health effects were related to nicotine‐containing e‐cigarettes, unless otherwise noted.
Health outcomes
Evidence regarding the health effects of e‐cigarettes is very limited. The risks of several adverse health outcomes are higher in e‐cigarette users. There is conclusive evidence that nicotine e‐cigarettes can cause poisoning and immediate inhalation toxicity (including seizures), particularly in children and adolescents, and that malfunctioning devices can cause injuries and burns; there is substantial evidence that nicotine e‐cigarettes can cause dependence or addiction in non‐smokers. There is conclusive evidence that e‐cigarettes cause e‐cigarette or vaping product use‐associated lung injury (EVALI), largely for e‐liquids containing THC (and the additive vitamin E acetate, identified in many, but not all, THC‐containing products); however, 14% of EVALI cases in the largest relevant study were linked to nicotine‐containing e‐liquids without these constituents.33 There is moderate evidence that nicotine e‐cigarettes can cause less serious adverse events, such as headache, cough, throat irritation, dizziness, and nausea. Identified environmental effects include waste, fires, and generation of indoor airborne particulate matter (substantial to conclusive evidence) (Box 3).
There is insufficient evidence regarding changes in respiratory symptoms, exacerbations of respiratory disease, lung function, and other respiratory measures in smokers who switch to exclusively using nicotine e‐cigarettes. There is limited or insufficient evidence that nicotine e‐cigarette use by non‐smokers (mostly people who had never smoked) leads to acute reductions in lung function or other respiratory measures. There is moderate evidence that nicotine e‐cigarettes immediately increase heart rate, systolic and diastolic blood pressure, and arterial stiffness acutely after use, by smokers.
Evidence is insufficient or unavailable regarding the effects of nicotine and non‐nicotine e‐cigarette use on cardiovascular disease, cancer, respiratory conditions other than lung injury, mental health, development in children and adolescents, reproduction, sleep, wound healing, neurological conditions (other than seizures), and endocrine, olfactory, ocular, allergic, and haematological conditions (Box 2, Box 3).
We rated the certainty of evidence for clinical and subclinical outcomes as low or very low, largely because of limitations in sample size and study design, and an overall paucity of evidence that caused serious concerns about inconsistency, imprecision, and indirectness (Supporting Information, part 5).
Smoking behaviour
Meta‐analyses of 25 longitudinal studies found strong evidence that young never‐smokers and non‐smokers who use e‐cigarettes are about three times as likely as non‐users to start smoking tobacco and to become regular smokers.18 Significant elevations in risk were identified by all studies included in the meta‐analyses. Potential residual confounding cannot be excluded, but the findings were similar after adjusting for a variety of factors, including risk‐taking. The relationship between e‐cigarette use and smoking was deemed likely to be causal according to the Bradford–Hill criteria.35
There is limited evidence from eleven randomised controlled trials that freebase nicotine e‐cigarettes used with clinical support are as efficacious as smoking cessation aids as approved nicotine replacement therapy or usual care/no intervention; evidence comparing their efficacy with that of non‐nicotine e‐cigarettes is insufficient.15,19 Evidence that non‐nicotine e‐cigarettes are efficacious compared with counselling or approved nicotine replacement therapy for smoking cessation is insufficient. There is limited evidence that the likelihood of relapse is about twice as high for ex‐smokers who use e‐cigarettes than for those who do not18 (Box 4).
Discussion
The published evidence indicates that use of nicotine e‐cigarettes increases the risks of adverse health outcomes, including addiction, poisoning, toxicity from inhalation (including seizures), and lung injury (largely but not exclusively attributable to THC/vitamin E acetate‐containing products). Devices can cause trauma and burns, largely because of exploding lithium batteries. There is evidence for adverse effects on cardiovascular health measures (including blood pressure and heart rate) and lung function. Non‐smoking young people who use e‐cigarettes are about three times as likely as non‐users to start tobacco smoking and to become regular smokers.18 Bearing in mind caveats regarding inferences based on observational data and the possibility of residual confounding, this relationship was deemed probably causal. Non‐smokers and young people are most vulnerable to e‐cigarette adverse events, as they are disproportionately affected by risks such as addiction, poisoning, toxicity from inhalation, and increased smoking uptake, with little or no potential benefit through increased smoking cessation.13 The impact of e‐cigarettes on the environment includes indoor air pollution, waste, and fires.
Evidence for the efficacy of freebase nicotine e‐cigarettes as smoking cessation aids was largely from studies incorporating clinical support and was limited. This conclusion is consistent with those of other major independent reviews (2018–2021) that evidence for the efficacy of e‐cigarettes as smoking cessation aids is limited,3 unavailable,9 similar to that of other smoking cessation therapies,36 inadequate,37 of increasing strength that they may be helpful in the short term,26 weak,2 or insufficient.38 The 2022 Cochrane review of e‐cigarettes as smoking cessation aids39 included major reviews published since our umbrella review search. We rated it less independent than other reviews because four of its authors were also investigators in the included trials. The review found high certainty evidence that nicotine e‐cigarettes were more efficacious as smoking cessation aids than standard nicotine replacement therapy (six studies), moderate certainty evidence that they were more efficacious than non‐nicotine e‐cigarettes, and very low certainty evidence that they were more efficacious than usual care or behavioural support.39 The review inclusion criteria were broader than ours, including self‐reported cessation outcomes and trials not published as peer‐reviewed articles, and the authors generally rated risk of bias in individual trials lower than we did.
A key finding of our synthesis was the general paucity of evidence for the effects of nicotine and non‐nicotine e‐cigarettes on many major clinical outcomes, including cancer, cardiovascular, metabolic, mental health, developmental, reproductive, and neurological outcomes (other than seizures). The evidence chiefly concerns nicotine e‐cigarettes and outcomes that can be detected within months or years of commencing use (including effects on smoking behaviour) and acute outcomes for which links with e‐cigarette use may be apparent at the individual or group level, such as addiction, poisoning, burns, nicotine toxicity, and EVALI. An English review we identified after our umbrella review search noted the lack of direct evidence with regard to major clinical outcomes and that most serious adverse events were infrequent; the authors focused on comparing the effects of e‐cigarette use with those of smoking and on biomarkers, and generally found more favourable results for e‐cigarettes than for tobacco cigarettes.40
Overall, our findings add to and concur broadly with those of other major reviews, including our conclusion that the direct impact of e‐cigarettes on clinical disease outcomes are largely unknown.
E‐cigarette users inhale a complex mixture of chemicals, including nicotine, solvent carriers, flavourings, tobacco‐specific nitrosamines, volatile organic compounds, phenolic compounds, tobacco alkaloids, aldehydes, free radicals, reactive oxygen species, furans, and metals; many are associated with adverse health effects.13,41 As well as being highly addictive, human and animal studies indicate that nicotine has adverse effects on cardiovascular markers, lung function, and brain development and function in adolescents.3,42,43,44,45,46,47,48,49 Certain harms identified here, including those related to devices, probably also apply to non‐nicotine e‐cigarettes, as does the uncertainty regarding other effects.
We identified a range of health harms, and no benefits, for non‐smokers who use e‐cigarettes. The World Health Organization recognises addiction per se as a harmful outcome.50 Nicotine addiction and aggressive marketing underpin the widespread and increasing use of e‐cigarettes by young people.51 The direct health risks, the association of e‐cigarette use with taking up tobacco smoking, and the uncertainty about their effects on major health outcomes mean that e‐cigarette use by non‐smokers, especially children and adolescents, is an important public health problem. The health impact of e‐cigarettes in ex‐smokers is reduced if they use other means to quit or if any e‐cigarette use is short‐term; there is limited evidence that relapse to smoking is more likely for e‐cigarette users. For non‐nicotine e‐cigarettes, we found no benefits in terms of smoking cessation, harms related to devices, and uncertainty regarding health effects, indicating overall harm.
Appropriate therapeutic use of a product requires evidence of acceptable safety and efficacy, a favourable risk–benefit balance, and quality.52,53,54 Most smokers who successfully quit do so without cessation aids,55,56 and many approved smoking cessation aids of established safety, quality, and efficacy are available.16 In many countries, dual tobacco smoking and e‐cigarette use is the most frequent pattern of e‐cigarette use.8,57,58 The direct health impact of dual use is unknown, and e‐cigarettes may facilitate continued smoking, increasing risks.59 Smokers are subject to the adverse health effects of e‐cigarettes, while other consequences (poisoning, environmental impact, use by non‐smokers) can affect family and community members. E‐cigarettes may be beneficial for smokers who use them to completely and promptly quit smoking, but the limited evidence in this regard, their risks, uncertainty about their effects on major clinical outcomes, and continued smoking by most users render their overall safety and efficacy unclear. They are not registered therapeutic goods in Australia or overseas.12 The United States Preventive Services Task Force noted that “the current evidence is insufficient to assess the balance of benefits and harms of electronic cigarettes (e‐cigarettes) for tobacco cessation in adults, including pregnant persons”38 and they are not currently recommended for this purpose in the United States.60 The RACGP, in light of the evidence limitations, recommends that e‐cigarettes be prescribed only for fully informed people who have unsuccessfully tried other smoking cessation methods.16
Risks related to e‐cigarettes are likely to be increased by certain product attributes, as well as by factors that lead to greater use by people not using them for smoking cessation, including higher e‐liquid nicotine concentration; greater e‐liquid volume; “at home” dilution and other e‐liquid preparation; adulteration of e‐liquids; poor labelling and non‐child‐resistant packaging; high concentration nicotine salt products; flavourings and other characteristics or branding that appeal to children, adolescents and non‐smokers; availability, advertising and promotion; low cost; inadequate enforcement of legal restrictions on the supply and advertising of e‐cigarettes; tobacco and nicotine industry influence; and misinformation about their effects on health.2,13,61,62
Limitations
Methodological challenges contribute to the paucity of reliable evidence. E‐cigarettes are relatively new, diverse, and changing rapidly.2 Consequently, long term evidence is unavailable and products examined in specific studies do not necessarily reflect current options, as indicated, for example, by the limited information on more recent nicotine salt products. Further evaluation of the effects of e‐cigarettes on health must consider product diversity and changes over time. Identified risks should be assumed to apply to e‐cigarettes in general, unless robust evidence indicates otherwise.
Most publications did not include information on e‐cigarette type or e‐liquid constituents, and e‐cigarettes labelled as nicotine‐free often include nicotine, undermining the reliability of some data.2 While the use of non‐nicotine e‐cigarettes is likely to be uncommon, their inclusion in some studies would reduce the apparent magnitude of nicotine‐related effects and our findings would consequently be conservative.
Reliable evidence on clinical health outcomes requires appropriate short and long term, large scale, high quality studies and statistical analyses. Moreover, the effects of e‐cigarettes and tobacco smoking must be reliably distinguished. Tobacco smoking causes a wide range of health harms that vary according to the intensity, duration, and recentness of smoking.63,64 Older e‐cigarette users are generally established and continuing smokers (dual users), smokers aiming to quit, and ex‐smokers, but younger users are often not established smokers, including people who have never smoked, have tried smoking in the past, or smoke infrequently. Confounding between the effects of e‐cigarette use and smoking is therefore a major problem, and statistical adjustment is unlikely to fully overcome this difficulty, particularly for the health conditions most affected by smoking. Consequently, people who have never smoked comprise the most reliable group in which to quantify the effects of e‐cigarettes, and people neither smoking nor using e‐cigarettes should be the main comparison group.3 Statements that e‐cigarettes are “safer than smoking” and terms such as “tobacco harm reduction” may be useful when discussing the minority of smokers who use e‐cigarettes to quit completely, but are not applicable to non‐smokers. Comparisons with the effects of smoking do not permit the reliable ascertainment of the direct health impact of e‐cigarettes.
For completeness, we also discussed evidence related to physiological and other outcome types and study designs. Findings from these study types should be interpreted cautiously as they do not provide reliable evidence about the causal relationships between e‐cigarette use and clinical disease outcomes.
The certainty of evidence was rated very low for most clinical and subclinical outcomes. As the tobacco and related industries can influence research conduct and distort study findings,65 it is important to ensure that research is free of competing interests. Risk of bias assessment was based on methodological quality and potential competing interests, such as financial support from the tobacco or pharmaceutical industries. Although risks of bias were noted across health outcomes, significant concerns in other domains meant that they were unlikely to have large effects on our interpretation of their findings or the overall certainty of evidence. Application of the precautionary principle, whereby exposure to an agent with uncertain effects should be avoided,66 is appropriate when serious adverse impacts are scientifically plausible, particularly those affecting future generations.
Conclusions
There is strong or conclusive evidence that nicotine e‐cigarettes can be harmful to health, particularly for non‐smokers and children and adolescents. Evidence for their effects, and those of non‐nicotine e‐cigarettes, on most important health outcomes is uncertain. Smokers who quit by switching completely and promptly to e‐cigarettes may benefit from their use, but they are not currently approved as medical smoking cessation aids. The evidence we have synthesised supports policy and regulatory efforts to reduce e‐cigarette use in the general population, particularly by non‐smokers and children, adolescents, and young adults, and for purposes other than smoking cessation. Better quality evidence is required regarding e‐cigarette health effects, their safety and efficacy as smoking cessation aids, and effective regulatory options.
Box 1 – Selection of publications for inclusion in the top‐up systematic review*
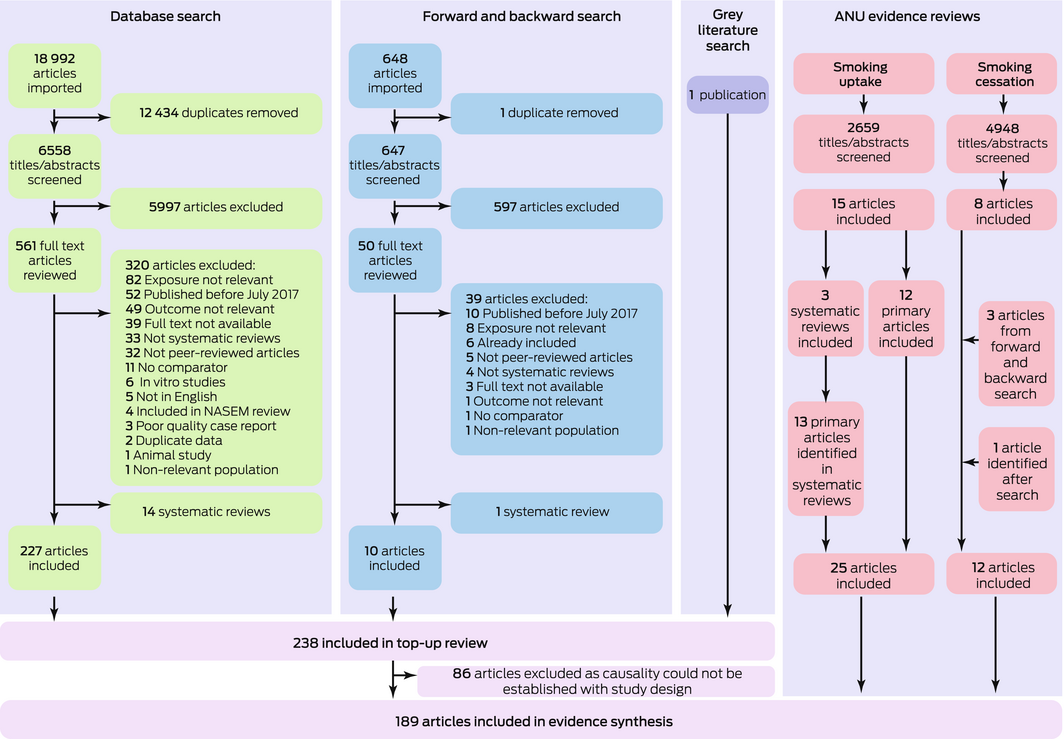
ANU = Australian National University. * Figure modified from our larger e‐cigarette health outcomes review report, with permission.13
Box 2 – Overview of the 400 publications included in our systematic review evidence synthesis, by health outcome category and study design*
|
Health outcome |
Meta‐analysis |
Randomised controlled trial |
Cohort study |
Non‐randomised intervention study |
Case–control study |
Surveillance report |
Cross‐sectional survey |
Case series |
Case report |
||||||
|
|
|||||||||||||||
|
Dependence and abuse liability |
|
13† |
1 |
17 |
|
|
21 |
|
|
||||||
|
Cardiovascular disease |
1 |
11 |
1 |
7 |
|
|
|
|
1 |
||||||
|
Cancer |
|
|
1 |
|
|
|
|
|
|
||||||
|
Respiratory disease |
|
10‡ |
6‡ |
8 |
|
18 |
|
7 |
9 |
||||||
|
Oral health |
|
|
2 |
2 |
|
|
|
|
|
||||||
|
Developmental/ reproductive |
|
|
2 |
|
|
|
1 |
|
|
||||||
|
Burns and injuries |
|
|
|
|
|
7 |
|
26 |
34 |
||||||
|
Poisoning |
|
|
|
|
|
28 |
|
4 |
27 |
||||||
|
Mental health |
|
|
3 |
|
|
|
|
|
|
||||||
|
Environmental hazards§ |
|
|
|
17 |
|
3 |
|
5 |
|
||||||
|
Neurological |
|
|
|
|
|
3 |
|
2 |
8 |
||||||
|
Sleep |
|
|
|
|
|
|
|
|
|
||||||
|
Less serious adverse events |
1 |
24 |
17 |
4 |
|
1 |
|
|
|
||||||
|
Ocular health |
|
|
|
1 |
|
|
|
|
|
||||||
|
Wound healing |
|
|
|
|
|
|
|
|
|
||||||
|
Olfactory |
|
|
|
|
|
|
1 |
|
|
||||||
|
Endocrine |
|
|
|
|
|
|
2 |
|
|
||||||
|
Allergy diseases |
|
|
|
|
|
|
|
1 |
3 |
||||||
|
Haematological |
|
|
|
|
|
|
|
|
|
||||||
|
Smoking uptake |
3 |
2 |
23 |
|
|
|
|
|
|
||||||
|
Smoking cessation |
|
11 |
|
|
|
|
|
|
|
||||||
|
|
|||||||||||||||
|
* Figure modified from our larger e‐cigarette health outcomes review report, with permission.13 In two cases, the listed study design is different to the design stated by the study authors. For example, a trial described by study authors as a “randomised crossover trial” without reference to randomisation did not meet the requirements to be classified a randomised trial. † One article described two separate randomised controlled trials,27 both of which are included in the count. ‡ Two article pairs that reported the same data (a cohort study28,29 and a randomised controlled trial30,31) were each combined for evidence synthesis. § Characterisation of studies with environmental outcomes differ from those for other outcomes; in this table, “non‐randomised intervention studies” refer to controlled experimental studies, “case series” to natural experiments. |
|||||||||||||||
Box 3 – Summary of evidence synthesis on relationships between use of e‐cigarettes and health outcomes*
|
Health outcome type (number of studies) |
Summary of conclusions from evidence review |
||||||||||||||
|
|
|||||||||||||||
|
Dependence and abuse liability (52) |
|
||||||||||||||
|
Cardiovascular disease (21) |
|
||||||||||||||
|
Cancer (1) |
|
||||||||||||||
|
Respiratory disease (58) |
|
||||||||||||||
|
Oral health (4) |
|
||||||||||||||
|
Developmental and reproductive (3) |
|
||||||||||||||
|
Burns and injuries (67) |
|
||||||||||||||
|
Poisoning (59) |
|
||||||||||||||
|
Mental health (3) |
|
||||||||||||||
|
Environmental hazards with health implications (25) |
|
||||||||||||||
|
Neurological (13) |
|
||||||||||||||
|
Sleep (0) |
|
||||||||||||||
|
Less serious adverse events (47) |
|
||||||||||||||
|
Ocular health (1) |
|
||||||||||||||
|
Wound healing (0) |
|
||||||||||||||
|
Olfactory (1) |
|
||||||||||||||
|
Endocrine (2) |
|
||||||||||||||
|
Allergic diseases (4) |
|
||||||||||||||
|
Haematological (0) |
|
||||||||||||||
|
|
|||||||||||||||
|
EVALI = e‐cigarette or vaping product use‐associated lung injury; THC = tetrahydrocannabinol. * Table modified from our larger e‐cigarette health outcomes review report, with permission.13 † Nicotine content not reported in original studies. Many studies did not provide data on whether products were nicotine or non‐nicotine e‐cigarettes; in such cases, use was assumed to be largely of nicotine e‐cigarettes. When the information was provided, the product types are specified. |
|||||||||||||||
Box 4 – Summary of evidence synthesis on relationships between e‐cigarette use and smoking behaviour*
|
Smoking behaviour (number of studies) |
Summary of conclusions from evidence review |
||||||||||||||
|
|
|||||||||||||||
|
Smoking uptake (28) |
|
||||||||||||||
|
Smoking and nicotine cessation (11) |
|
||||||||||||||
|
|
|||||||||||||||
|
* Table modified from our larger e‐cigarette health outcomes review report, with permission. 13 Many studies did not provide data on whether products were nicotine or non‐nicotine e‐cigarettes; in these cases, use was assumed to be largely of nicotine e‐cigarettes. When the information was provided, the product types are specified. |
|||||||||||||||
Received 29 September 2022, accepted 27 February 2023
- Emily Banks1
- Amelia Yazidjoglou1
- Sinan Brown1
- Mai Nguyen1
- Melonie Martin1
- Katie Beckwith1
- Amanda Daluwatta1
- Sai Campbell1
- Grace Joshy1
- National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT
Open access
Open access publishing facilitated by Australian National University, as part of the Wiley ‐ Australian National University agreement via the Council of Australian University Librarians.
This systematic review and meta‐analysis19 was conducted as part of an independent program examining the health impacts of e‐cigarettes, funded by the Australian Department of Health and the National Health and Medical Research Council (NHMRC). Emily Banks is supported by an NHMRC Principal Research Fellowship (1136128).
The report on which this article is based13 was reviewed by members of the NHMRC Electronic Cigarettes Working Committee and staff at the Australian Department of Health. It was subject to an independent methodological review as a part of standard NHMRC processes.
We are grateful to the authors of Australian National University reports who contributed to this document, including Olivia Baenziger, Laura Ford, Miranda Harris, Tehzeeb Zulfiqar, and Robyn Lucas. We also acknowledge the expert input of the NHMRC Electronic Cigarettes Working Committee. We are grateful to staff at the NHMRC and the Australian Department of Health for their engagement as stakeholders, including regarding the scope of the review. We acknowledge Christine McDonald (Austin Health), Sotiris Vardoulakis (Australian National University), Matthew Peters (Macquarie University and University of Sydney), and Jessamine Soderstrom (Royal Perth Hospital) for their expert reviews of sections of the large report.13
No relevant disclosures.
- 1. US Department of Health and Human Services. E‐cigarette use among youth and young adults: a report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. https://www.ncbi.nlm.nih.gov/books/NBK538680/pdf/Bookshelf_NBK538680.pdf (viewed Nov 2019).
- 2. Scientific Committee on Health, Environmental and Emerging Risks (SCHEER). Opinion on electronic cigarettes. 16 Apr 2021. https://health.ec.europa.eu/system/files/2022‐08/scheer_o_017.pdf (viewed Sept 2021).
- 3. Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems (National Academies of Sciences, Engineering, and Medicine). Public health consequences of e‐cigarettes. Washington (DC): National Academies Press, 2018. https://www.ncbi.nlm.nih.gov/books/NBK507171/pdf/Bookshelf_NBK507171.pdf (viewed Nov 2019).
- 4. Hajek P, Przulj D, Phillips A, et al. Nicotine delivery to users from cigarettes and from different types of e‐cigarettes. Psychopharmacology (Berl) 2017; 234: 773‐779.
- 5. Yingst JM, Foulds J, Veldheer S, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS One 2019; 14: e0220300.
- 6. The Tobacco Atlas. E‐cigarettes & HTPs. Updated 18 May 2022. https://tobaccoatlas.org/challenges/e‐cigarettes‐htps (viewed Aug 2022).
- 7. Yoong SL, Hall A, Leonard A, et al. Prevalence of electronic nicotine delivery systems and electronic non‐nicotine delivery systems in children and adolescents: a systematic review and meta‐analysis. Lancet Public Health 2021; 6: e661‐e673.
- 8. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2019 (Cat. no. PHE 270): tobacco smoking supplementary data tables. 16 July 2020. https://www.aihw.gov.au/reports/illicit‐use‐of‐drugs/national‐drug‐strategy‐household‐survey‐2019/data (viewed Feb 2021).
- 9. Byrne S, Brindal E, Williams G, et al. E‐cigarettes, smoking and health: a literature review update. 22 June 2018. https://www.csiro.au/en/research/health‐medical/diseases/Health‐impacts‐of‐electronic‐cigarettes (viewed Nov 2019).
- 10. McCarthy A, Lee C, O'Brien D, et al. Harms and benefits of e‐cigarettes and heat‐not‐burn tobacco products: a literature map. June 2020. https://www.hrb.ie/fileadmin/2._Plugin_related_files/Publications/2020_publication‐related_files/2020_HIE/Evidence_Centre/Harms_and_benefits_of_e‐cigarettes_and_heat‐not‐burn_tobacco_products_Literature_map.pdf (viewed Feb 2021).
- 11. McNeill A, Brose LS, Calder R, et al. Evidence review of e‐cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. Feb 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/684963/Evidence_review_of_e‐cigarettes_and_heated_tobacco_products_2018.pdf (viewed Nov 2019).
- 12. Patnode CD, Henderson JT, Melnikow J, et al. Interventions for tobacco cessation in adults, including pregnant persons: an evidence update for the US Preventive Services Task Force (Agency for Healthcare Research and Quality publication no. 20‐05264‐EF‐1). Jan 2021. https://www.ncbi.nlm.nih.gov/books/NBK567066/pdf/Bookshelf_NBK567066.pdf (viewed July 2021).
- 13. Banks E, Yazidjoglou A, Brown S, et al. Electronic cigarettes and health outcomes: systematic review of global evidence. Report for the Australian Department of Health. 7 Apr 2022. https://openresearch‐repository.anu.edu.au/handle/1885/262914 (viewed Apr 2022).
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71.
- 15. Yazidjoglou A, Ford L, Baenziger O, et al. Efficacy of e‐cigarettes as aids to cessation of combustible tobacco smoking: updated evidence review. Final report prepared for the Australian Government Department of Health. 14 Sept 2021. https://openresearch‐repository.anu.edu.au/bitstream/1885/247864/1/Updated%20Evidence%20Review%20EC%20and%20Cessation%20for%20online%20publication%20210915.pdf (viewed Sept 2021).
- 16. Royal Australian College of General Practitioners. Pharmacotherapy for smoking cessation. In: Supporting smoking cessation: a guide for health professionals. Updated 29 Sept 2021. Melbourne: RACGP, 2019; pp. 31‐53. https://www.racgp.org.au/getattachment/c07241b0‐9dc1‐41bd‐b25b‐764389a675c9/Pharmacotherapy‐for‐smoking‐cessation.aspx (viewed Aug 2022).
- 17. National Health and Medical Research Council. 2022 CEO statement on electronic cigarettes. 2022. https://www.nhmrc.gov.au/health‐advice/all‐topics/electronic‐cigarettes/ceo‐statement (viewed Aug 2022).
- 18. Baenziger O, Ford L, Yazidjoglou A, et al. E‐cigarette use and combustible tobacco cigarette smoking uptake among non‐smokers, including relapse in former smokers: umbrella review, systematic review and meta‐analysis. BMJ Open 2021; 11: e045603.
- 19. Banks E, Beckwith K, Joshy G. Summary report on use of e‐cigarettes and relation to tobacco smoking uptake and cessation, relevant to the Australian context. 24 Sept 2020. https://openresearch‐repository.anu.edu.au/bitstream/1885/211618/3/E‐cigarettes%20smoking%20behaviour%20summary%20report%20final%20200924.pdf (viewed Sept 2020).
- 20. Ahmed N, Kalininskiy A, Gandhi H, Shin JJ. Spontaneous coronary artery dissection in a postpartum e‐cigarette smoker. BMJ Case Rep 2018; 2018: bcr2018225194.
- 21. Holland TE, De La Feld S. Cigarette dermatitis. Dermatitis 2019; 30: 272.
- 22. Simpson LJ, Lye G. Burns injuries from e‐cigarettes kept in pockets. BMJ 2019; 364: l554.
- 23. Schünemann H, Brozek J, Guyatt G, Oxman A (editors). GRADE handbook. Updated Oct 2013. https://gdt.gradepro.org/app/handbook/handbook.html (viewed July 2021).
- 24. Harder T, Sin MA, Bosch‐Capblanch X, et al. Towards a framework for evaluating and grading evidence in public health. Health Policy 2015; 119: 732‐736.
- 25. McNeill A, Brose LS, Calder R, et al. Vaping in England: an evidence update including mental health and pregnancy, March 2020. A report commissioned by Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/869401/Vaping_in_England_evidence_update_March_2020.pdf (viewed June 2020).
- 26. McNeill A, Brose L, Calder R, et al. Vaping in England: an evidence update including vaping for smoking cessation, February 2021. A report commissioned by Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/962221/Vaping_in_England_evidence_update_February_2021.pdf (viewed Mar 2021).
- 27. Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an e‐cigarette. Nicotine Tob Res 2016; 18: 1588‐1595.
- 28. Polosa R, Morjaria JB, Caponnetto P, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 2016; 21: 99‐108.
- 29. Polosa R, Morjaria J, Caponnetto P, et al. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. Int J Environ Res Public Health 2014; 11: 4965‐4977.
- 30. Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1‐year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46: 698‐706.
- 31. Cibella F, Campagna D, Caponnetto P, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond) 2016; 130: 1929‐1937.
- 32. Romberga AR, Miller Lo EJ, Cuccia AF, et al. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013–2018. Drug Alcohol Depend 2019; 203: 1–7.
- 33. Krishnasamy VP, Hallowell BD, Ko JY, et al; Lung Injury Response Epidemiology/Surveillance Task Force. Update: characteristics of a nationwide outbreak of e‐cigarette, or vaping, product use‐associated lung injury, United States, August 2019 – January 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 90‐94.
- 34. Govindarajan P, Spiller HA, Casavant MJ, et al. E‐cigarette and liquid nicotine exposures among young children. Pediatrics 2018; 141: e20173361.
- 35. Hill AB. The environment and disease: association or causation? J R Soc Med 1965; 58: 295‐300.
- 36. Quigley J, Kennelly H, Lee C, et al. Electronic cigarettes and smoking cessation: an evidence review. June 2020. https://www.hrb.ie/fileadmin/2._Plugin_related_files/Publications/2020_publication‐related_files/2020_HIE/Evidence_Centre/Electronic_cigarettes_and_smoking_cessation_systematic_evidence_review.pdf (viewed Sept 2021).
- 37. US Department of Health and Human Services. Smoking cessation. A report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, 2020. https://www.hhs.gov/sites/default/files/2020‐cessation‐sgr‐full‐report.pdf (viewed July 2020).
- 38. US Preventive Services Task Force. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force Recommendation Statement. JAMA 2021; 325: 265‐279.
- 39. Hartmann‐Boyce J, Lindson N, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2022; 11: CD010216.
- 40. McNeill A, Simonavičius E, Brose LS, et al. Nicotine vaping in England: 2022 evidence update. A report commissioned by the Office for Health Improvement and Disparities. 29 Sept 2022. https://www.gov.uk/government/publications/nicotine‐vaping‐in‐england‐2022‐evidence‐update/nicotine‐vaping‐in‐england‐2022‐evidence‐update‐main‐findings (viewed Oct 2022).
- 41. Australian Department of Health. National Industrial Chemicals Notification and Assessment Scheme. Non‐nicotine liquids for e‐cigarette devices in Australia: chemistry and health concerns. 2 Oct 2019. https://www.industrialchemicals.gov.au/sites/default/files/2020‐08/Non‐nicotine%20liquids%20for%20e‐cigarette%20devices%20in%20Australia%20chemistry%20and%20health%20concerns%20%5BPDF%201.21%20MB%5D.pdf (viewed Oct 2019).
- 42. US Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf (viewed July 2021).
- 43. Benowitz NL. Pharmacology of nicotine: addiction, smoking‐induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2009; 49: 57‐71.
- 44. Cowperthwaite B, Hains SMJ, Kisilevsky BS. Fetal behavior in smoking compared to non‐smoking pregnant women. Infant Behav Dev 2007; 30: 422‐430.
- 45. Jacobsen LK, Slotkin TA, Mencl WE, et al. Gender‐specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology 2007; 32: 2453‐2464.
- 46. Paus T, Nawazkhan I, Leonard G, et al. Corpus callosum in adolescent offspring exposed prenatally to maternal cigarette smoking. NeuroImage 2008; 40: 435‐441.
- 47. Quaranta L, Sabatelli M, Madia F, et al. Expanding the nosology of hypermyelinating neuropathies: description of two new entities [abstract]. J Periph Nerv Syst 2002; 7: 82‐83.
- 48. Dao JM, McQuown SC, Loughlin SE, et al. Nicotine alters limbic function in adolescent rat by a 5‐HT1A receptor mechanism. Neuropsychopharmacology 2011; 36: 1319‐1331.
- 49. Shram MJ, Funk D, Li Z, Lê AD. Acute nicotine enhances c‐fos mRNA expression differentially in reward‐related substrates of adolescent and adult rat brain. Neurosci Lett 2007; 418: 286‐291.
- 50. World Health Organization. WHO report on the global tobacco epidemic, 2019: offer help to quit tobacco use. 25 July 2019. https://www.who.int/publications/i/item/9789241516204 (viewed Sept 2019).
- 51. Jankowski M, Krzystanek M, Zejda JE, et al. E‐cigarettes are more addictive than traditional cigarettes: a study in highly educated young people. Int J Environ Res Public Health 2019; 16: 2279.
- 52. European Medicines Agency. What we do. Undated. https://www.ema.europa.eu/en/about‐us/what‐we‐do (viewed July 2022).
- 53. Food and Drug Administration. What we do. Updated 28 Mar 2018. https://www.fda.gov/about‐fda/what‐we‐do (viewed July 2022).
- 54. Therapeutic Goods Administration. Product regulation according to risk. Undated. https://www.tga.gov.au/product‐regulation‐according‐risk (viewed July 2022).
- 55. Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med 2010; 7: e1000216.
- 56. Soulakova JN, Crockett LJ. Unassisted quitting and smoking cessation methods used in the United States: analyses of 2010–2011 tobacco use supplement to the current population survey data. Nicotine Tob Res 2016; 20: 30‐39.
- 57. Reid JL, Rynard VL, Czoli CD, Hammond D. Who is using e‐cigarettes in Canada? Nationally representative data on the prevalence of e‐cigarette use among Canadians. Prev Med 2015; 81: 180‐183.
- 58. Sung HY, Wang Y, Yao T, et al. Polytobacco use and nicotine dependence symptoms among US adults, 2012–2014. Nicotine Tob Res 2018; 20 (Suppl 1): S88‐S98.
- 59. WHO Scientific Advisory Committee on Tobacco Product Regulation; WHO Tobacco Free Initiative. SACTob statement of principles guiding the evaluation of new or modified tobacco products / Scientific Advisory Committee on Tobacco Product Regulation (SACTob). 2003. https://apps.who.int/iris/handle/10665/42648 (viewed June 2021).
- 60. Selby P, Zawertailo L. Tobacco addiction. N Engl J Med 2022; 387: 345‐354.
- 61. Australian Government. Therapeutic Goods (Standard for Nicotine Vaping Products) (TGO 110) Order 2021. 13 May 2021. https://www.legislation.gov.au/Series/F2021L00595 (viewed Nov 2021).
- 62. Government of Canada. Vaping products: new limits on nicotine concentration and consultation on flavour restrictions. Updated 21 June 2021. https://www.canada.ca/en/health‐canada/news/2021/06/backgrounder‐vaping‐products‐‐new‐limits‐on‐nicotine‐concentration‐and‐consultation‐on‐flavour‐restrictions.html (viewed Aug 2021).
- 63. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality‐beyond established causes. N Engl J Med 2015; 372: 631‐640.
- 64. Pirie K, Peto R, Reeves GK, et al; Million Women Study Collaborators. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013; 381: 133‐141.
- 65. World Health Organization. Tobacco industry interference with tobacco control. 22 May 2008. https://www.who.int/publications/i/item/9789241597340 (viewed July 2021).
- 66. World Commission on the Ethics of Scientific Knowledge and Technology. The precautionary principle. Paris: United Nations Educational, Scientific and Cultural Organization, 2005. https://unesdoc.unesco.org/ark:/48223/pf0000139578 (viewed June 2021).






Abstract
Objective: To review and synthesise the global evidence regarding the health effects of electronic cigarettes (e‐cigarettes, vapes).
Study design: Umbrella review (based on major independent reviews, including the 2018 United States National Academies of Sciences, Engineering, and Medicine [NASEM] report) and top‐up systematic review of published, peer‐reviewed studies in humans examining the relationship of e‐cigarette use to health outcomes published since the NASEM report.
Data sources: Umbrella review: eight major independent reviews published 2017–2021. Systematic review: PubMed, MEDLINE, Scopus, Web of Science, the Cochrane Library, and PsycINFO (articles published July 2017 – July 2020 and not included in NASEM review).
Data synthesis: Four hundred eligible publications were included in our synthesis: 112 from the NASEM review, 189 from our top‐up review search, and 99 further publications cited by other reviews. There is conclusive evidence linking e‐cigarette use with poisoning, immediate inhalation toxicity (including seizures), and e‐cigarette or vaping product use‐associated lung injury (EVALI; largely but not exclusively for e‐liquids containing tetrahydrocannabinol and vitamin E acetate), as well as for malfunctioning devices causing injuries and burns. Environmental effects include waste, fires, and generation of indoor airborne particulate matter (substantial to conclusive evidence). There is substantial evidence that nicotine e‐cigarettes can cause dependence or addiction in non‐smokers, and strong evidence that young non‐smokers who use e‐cigarettes are more likely than non‐users to initiate smoking and to become regular smokers. There is limited evidence that freebase nicotine e‐cigarettes used with clinical support are efficacious aids for smoking cessation. Evidence regarding effects on other clinical outcomes, including cardiovascular disease, cancer, development, and mental and reproductive health, is insufficient or unavailable.
Conclusion: E‐cigarettes can be harmful to health, particularly for non‐smokers and children, adolescents, and young adults. Their effects on many important health outcomes are uncertain. E‐cigarettes may be beneficial for smokers who use them to completely and promptly quit smoking, but they are not currently approved smoking cessation aids. Better quality evidence is needed regarding the health impact of e‐cigarette use, their safety and efficacy for smoking cessation, and effective regulation.
Registration: Systematic review: PROSPERO, CRD42020200673 (prospective).