The known: Androgen deprivation therapy (ADT) is effective for treating prostate cancer, but it accelerates bone loss and increases fracture risk. Australian guidelines recommend dual‐energy x‐ray absorptiometry (DXA) scanning of men commencing ADT as part of baseline fracture risk assessment.
The new: The baseline bone health of fewer than 20% of Australian men with prostate cancer commencing ADT was assessed by DXA scanning from six months before to twelve months after initiation of therapy.
The implications: Health care professionals caring for men with prostate cancer starting ADT should ensure that their bone health is routinely assessed by DXA, as recommended by guidelines and subsidised for this purpose by the MBS.
Prostate cancer is the most frequently diagnosed malignancy in men; it was estimated that more than 24 000 Australian men would be diagnosed with it in 2022.1 Androgen deprivation therapy (ADT) is often used in men with prostate cancer, but can cause severe hypogonadism, which accelerates bone mineral density loss.2 Androgen deprivation therapy is associated with 3–5% annual decline in bone mineral density in men with prostate cancer, including those without skeletal metastases.3 In one American study, bone mineral density declined by 2.5% at the hip and 4.0% at the lumbar spine during the first twelve months of ADT, whereas it did not change significantly in men with prostate cancer not receiving ADT, nor in age‐matched healthy controls.4 Androgen deprivation therapy has a cumulative effect on bone mineral density. In non‐metastatic prostate cancer, osteoporosis — defined as a dual‐energy x‐ray absorptiometry (DXA) T‐score of –2.5 or less at any measurement site — was identified in 35.4% of ADT‐naïve men, in 42.9% after two years of ADT, in 49.2% after four years, and in 80.6% after ten or more years of ADT.5
ADT‐related bone mineral density loss is associated with increased fracture incidence. In one study, a larger proportion of men with prostate cancer receiving ADT had experienced fractures 12–60 months after diagnosis with prostate cancer than of those never treated with ADT (19.4% v 12.6%); the number needed to harm was 28 (95% confidence interval [CI], 26–31).3 A meta‐analysis similarly indicated that fracture risk was higher for men with prostate cancer treated with ADT than for those who were not (relative risk, 1.23; 95% CI, 1.10–1.38).6
Effective bone protection therapy is available for men with prostate cancer receiving ADT, including zoledronic acid,7,8,9 alendronate,10,11 and denosumab.12 Denosumab treatment, for instance, has been associated with reduced incidence of new vertebral fractures in men with prostate cancer receiving ADT.12
Although Australian13,14 and overseas guidelines15,16,17,18 recommend assessing bone health when commencing ADT in men with prostate cancer, reported DXA screening rates are poor.19,20 A Canadian study (33 036 patients) found that the rate of DXA bone mineral density assessment within two years of ADT initiation ranged between 0.5 per 100 person‐years in 1995 and 18.0 per 100 person‐years in 2008;19 an American study reported that only 197 of 2290 men with prostate cancer (8.6%) had undergone DXA scanning between twelve months before and six months after starting ADT.20
National Australian data on DXA screening of men commencing ADT have not been reported. We therefore examined the prevalence in Australia of DXA bone health assessment from six months before to twelve months after initial dispensing of ADT to men with prostate cancer.
Methods
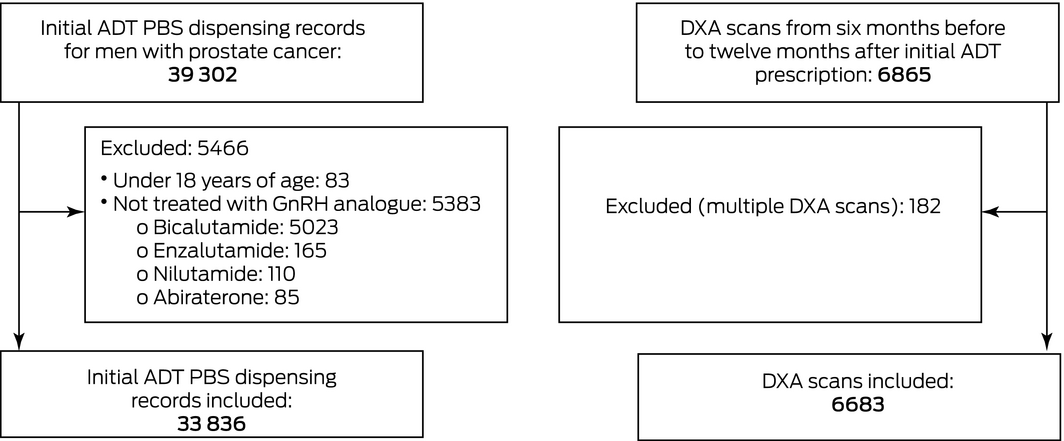
Men (18 years or older) with prostate cancer who commenced ADT during 1 May 2017 – 31 July 2020 were identified in the Pharmaceutical Benefits Scheme (PBS) database by PBS code (, table 1). DXA scans from six months before to twelve months after initial ADT dispensing were identified in the Medicare Benefits Schedule (MBS) database by MBS item number (, table 2). After linking the de‐identified PBS and MBS datasets using patient identifier codes, we also extracted socio‐demographic information (age, state of residence) for the included men.
Androgen deprivation therapy (ADT) was defined as treatment with a gonadotropin‐releasing hormone analogue. We excluded men who had commenced treatment with newer hormonal agents with other mechanisms of action (eg, bicalutamide, enzalutamide, nilutamide, and abiraterone alone). We included only the DXA scan performed closest to the date of ADT initiation.
Statistical analyses were performed in SPSS 27 and 28 (IBM) and MedCalc (https://www.medcalc.org). We report results as means with standard deviations (SDs). We estimated the proportions (with 95% CI) of men dispensed ADT who underwent DXA scanning, both overall and by age, state of residence, and ADT type. We examined the influence of these characteristics in multiple logistic regression analyses, and report adjusted odds ratios (aORs) with 95% CIs. P < 0.05 was deemed statistically significant (two‐tailed t tests).
Ethics approval
The Western Sydney Local Health District Human Research Ethics Committee approved our study as a low/negligible risk project (HREC 2019/ETH13066).
Results
A total of 33 836 initial PBS‐subsidised ADT prescriptions for men with prostate cancer were dispensed during 1 May 2017 – 31 July 2020 (Box 1). The mean age of the men was 75 years (SD, 7.9 years); 24 385 were aged 70 years or older (72.1%).
During the period from six months before to twelve months after first dispensing of ADT, 6683 men underwent DXA scanning (19.8%; 95% CI, 19.3–20.2%); the mean time from first ADT dispensing to DXA scan was +90 days (SD, 134 days), and was similar in all Australian states (data not shown). Among the 1470 men who had DXA scans prior to ADT initiation (22.0% of men with scans; 95% CI, 21.0–22.9%), the mean time between scan and ADT initiation was –77 days (SD, 57 days); among the 5212 men who had DXA scans after ADT initiation (78.0%; 95% CI, 77.1–78.9%), the mean time between ADT initiation and scan was +140 days (SD, 112 days).
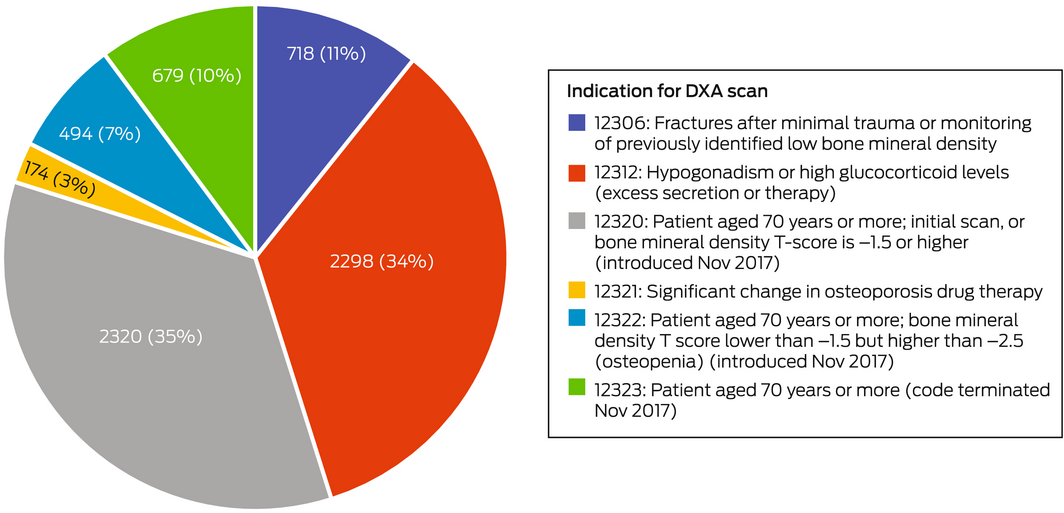
The MBS item indications for 3493 DXA scans (52%) were age‐related (ie, for men aged 70 years or more; MBS items 12320, 12322, 12323); the indication for 2298 scans (34%) was bone health assessment in men with hypogonadism or glucocorticoid excess (MBS item 12312) (Box 2). Age‐related scans comprised a larger proportion of scans before than after ADT initiation (936 of 1339, 69.9% v 2557 of 5344, 47.9%; , table 3), while item 12312 scans comprised a larger proportion of scans after than before ADT initiation (2120 of 5344, 39.7% v 178 of 1339, 13.3%).
The proportion of men aged 70–84 years who had DXA scans (23.4%) was larger than for younger men; men aged 54 years or less were least likely to have scans (proportion, 10%; v. 70–84 years: aOR, 0.36; 95% CI, 0.28–0.47). Larger proportions of Australian Capital Territory (33.7%), New South Wales (24.8%), and Northern Territory men with prostate cancer (23%) had DXA scans than in other states. Men receiving the most frequently prescribed ADT agent, goserelin (13 752 of 33 836 prescriptions, 40.6%), were less likely than men receiving other ADT agents to have scans (Box 3).
Among the 5320 DXA scans of men aged 70 years or more, 3416 (64%) were billed as age‐related MBS items (936 before [79% of pre‐initiation scans], 2480 after ADT initiation [60% of post‐initiation scans]) and 1281 as item 12312 scans (24%; 86 before [7% of pre‐initiation scans] and 1195 after ADT initiation [29% of post‐initiation scans]) (, tables 3 and 4).
Discussion
We report the first Australian prevalence study of DXA baseline bone health assessment in men with prostate cancer commencing ADT. Despite guideline recommendations13,14,15,16,17,18 and their eligibility for MBS‐subsided DXA scans under item number 12312, only 20% of men had DXA scans between six months before and twelve months after first dispensing of ADT.
In an earlier retrospective chart review of men with high risk prostate carcinoma who commenced adjuvant ADT between 1 January 2016 and 31 December 2017 at the Mid‐North Coast Cancer Institute, Coffs Harbour (the only site that provides specialist radiation therapy and integrated oncology services in the region), we found that 26 of 188 men (14%) had DXA scans before and 133 men (71%) after commencing ADT (median, +20 days; interquartile range, 7–98 days), overall a much higher proportion than reported in North America.20,21 Applying DXA criteria,19 76 assessed men (48%) had osteopenia (T‐score of –1.0 or less but higher than –2.5) and 38 (24%) osteoporosis (T‐score of –2.5 or lower).
By analysing national data in our new study, we reduced selection bias and expand the generalisability of our findings to the entire Australian population of men with prostate cancer commencing ADT. The study time frame chosen (2017–20) minimised the effects of seasonal events and the COVID‐19 pandemic. Australian guidelines recommend DXA bone mineral density assessment when initiating ADT,13 and a DXA result from the six months preceding initiation facilitates early bone protective therapy. The broad time frame before and after ADT initiation recognised pragmatic difficulties in commencing cancer treatment. The 12‐month post‐ADT initiation period was chosen because bone density falls during the first twelve months of ADT therapy.4
MBS‐subsidised DXA testing for men with prostate cancer commencing ADT may be underused for several reasons. We found that men under 70 years of age were less likely to have DXA scans than older men, suggesting that fracture risk in younger men is not recognised, or that physicians are not aware that men receiving ADT are eligible for MBS‐subsidised DXA scans. Only 2298 of 33 836 men (6.8%) had DXA scans with hypogonadism as an indication (MBS item number 12312). Geographic differences in DXA access may have depressed the DXA scanning rate;22 testing was more frequent in some jurisdictions with highly urbanised populations, such as NSW and the ACT.
Radiation and medical oncologist, urologist, and general practitioner professional bodies and consumer organisations such as Healthy Bones Australia (https://healthybonesaustralia.org.au; via the National Strategic Action Plan for Osteoporosis23) and the Prostate Cancer Foundation of Australia (https://www.pcfa.org.au) could increase awareness among patients and health care professionals of the effect of ADT on bone health and the availability of both MBS‐subsidised DXA scanning and bone protective therapy. Bone health should be considered part of routine care for men with prostate cancer receiving ADT. However, uncertainty about whether osteoporosis in men with cancer should be managed in primary or specialty care must be dispelled. Streamlined referral processes are needed at the local level to support timely DXA assessment and, if required, early initiation of bone protection therapy. This could be facilitated by clinical pathways including the patients, medical and radiation oncologists, urologists, general practitioners, rheumatologists, endocrinologists, physiotherapists, and nurses, and adapted according to locally available resources and expertise, particularly outside metropolitan centres.
A Canadian study described the development and implementation of BoneRx, a healthy bone prescription tool for improving bone health care and increasing awareness of bone health among men receiving ADT.24 The BoneRx tool was given to patients at the initiation of ADT and provided prompts for treating health care professionals and targeted education for the patient. The document included a “healthy bones prescription” that prompted the treating specialist to recommend DXA assessment, advice about calcium and vitamin D supplementation and physical activity, and the patient booklet, Building strong bones: for men taking androgen deprivation therapy. The intervention increased levels of calcium and vitamin D supplementation and exercise among the treated men.24
The frequency of osteoporotic fractures linked with iatrogenic hypogonadism in men treated with ADT will increase with the rising prevalence of prostate cancer (with excellent long term prognosis) in our ageing population. The baseline bone health of all men with prostate cancer, regardless of age or ADT type, should be assessed when ADT is initiated. A Markov state transition model indicated that five years’ treatment with alendronate was cost‐effective for men with osteoporosis and locally advanced or high risk localised prostate cancer commencing a two‐year ADT course after radiation therapy.25 In addition to DXA screening, we support guideline recommendations that ADT‐independent osteoporosis risk factors be assessed.2
Limitations
Our analysis did not consider the anticipated duration of ADT treatment, but men requiring longer courses would be at greater risk of bone mineral density loss and fracture.5 Further, the proportion of men prescribed bicalutamide as first PBS‐subsidised prostate cancer treatment but later prescribed an additional ADT medication was unknown. Nevertheless, only 746 of 5023 men who commenced treatment with bicalutamide had DXA scans (14.9%), and a sensitivity analysis including these patients yielded similar findings (data not shown). Inpatient and self‐funded DXA scans (ie, not MBS‐funded) were not included in our analysis. Men with histories of osteoporosis or skeletal metastases may not have had DXA scans during the study window but could have already commenced protective therapy (a bisphosphonate or denosumab). Moreover, as men aged 70 years or more could have had DXA scans for MBS‐specified indications other than hypogonadism (including age), we may have overestimated the prevalence of DXA scans undertaken specifically to assess bone density at the commencement of ADT.
Conclusions
Despite evidence‐based guidelines and the availability of bone protective therapy, 80% of Australian men with prostate cancer commencing ADT do not undergo DXA screening. ADT‐related bone loss and fracture will be increasingly common problems as our population ages, the prevalence of prostate cancer increases, and its long term prognosis improves. Educating general practitioners, radiation and medical oncologists, urologists, rheumatologists, endocrinologists, geriatricians, physiotherapists, nurses, and the general public could improve the assessment and management of long term bone health in men with prostate cancer. Clinical pathways that embed bone health management in their routine care should be developed.
Box 1 – Dual‐energy x‐ray absorptiometry (DXA) scans for men with prostate cancer commencing androgen deprivation therapy (ADT) in Australia, 1 May 2017 – 31 July 2020
GnRH = gonadotropin‐releasing hormone.
Box 2 – Dual‐energy x‐ray absorptiometry (DXA) scans of 6683 men between six months before and twelve months after initiating androgen deprivation therapy (ADT), by Medicare Benefits Schedule (MBS) item numbers
Box 3 – Dual‐energy x‐ray absorptiometry (DXA) assessment of men with prostate cancer from six months before to twelve months after initiation of androgen deprivation therapy (ADT), by age, residential state, and ADT medication
Men starting ADT with DXA scans |
Odds ratio (95% CI) | ||||||||||||||
Characteristic |
Men starting ADT |
Number |
Proportion (95% CI) |
Unadjusted |
Adjusted* | ||||||||||
Total number of men |
33 836 |
6683 |
19.8% (19.3–20.2%) |
||||||||||||
Age |
|||||||||||||||
54 years or younger |
639 (1.9%) |
66 |
10% (8.1–13%) |
0.38 (0.29–0.49) |
0.36 (0.28–0.47) |
||||||||||
55–69 years |
8812 (26.0%) |
1297 |
14.7% (14.0–15.5%) |
0.57 (0.53–0.61) |
0.56 (0.52–0.60) |
||||||||||
70–84 years |
19 378 (57.3%) |
4528 |
23.4% (22.8–24.0%) |
1 |
— |
||||||||||
85 years or older |
5007 (14.8%) |
792 |
15.8% (14.8–16.9%) |
0.62 (0.57–0.67) |
0.61 (0.57–0.67) |
||||||||||
State of residence |
— |
||||||||||||||
Australian Capital Territory |
410 (1.2%) |
138 |
33.7% (28.9–38.2%) |
1.52 (1.23–1.88) |
1.54 (1.24–1.90) |
||||||||||
New South Wales |
11 102 (32.8%) |
2752 |
24.8% (24.0–25.6%) |
1 |
|||||||||||
Northern Territory |
178 (0.5%) |
41 |
23% (17–30%) |
0.91 (0.64–1.29) |
0.98 (0.68–1.39) |
||||||||||
Queensland |
7478 (22.1%) |
1365 |
18.3% (17.3–19.1%) |
0.68 (0.63–0.73) |
0.66 (0.62–0.71) |
||||||||||
South Australia |
3052 (9.0%) |
446 |
14.6% (13.4–16.0%) |
0.52 (0.47–0.58) |
0.53 (0.48–0.59) |
||||||||||
Tasmania |
789 (2.3%) |
114 |
14.4% (12.0–17.0%) |
0.51 (0.41–0.62) |
0.47 (0.39–0.59) |
||||||||||
Victoria |
7841 (23.2%) |
1494 |
19.1% (18.1–19.9%) |
0.71 (0.66–0.77) |
0.73 (0.68–0.78) |
||||||||||
Western Australia |
2986 (8.8%) |
333 |
11.2% (10.1–12.4%) |
0.38 (0.34–0.43) |
0.38 (0.34–0.43) |
||||||||||
ADT medication |
|||||||||||||||
Goserelin |
13 752 (40.6%) |
2376 |
17.3% (16.7–17.9%) |
1 |
— |
||||||||||
Leuprorelin |
13 312 (39.3%) |
2793 |
21.0% (20.3–21.7%) |
1.27 (1.20–1.35) |
1.18 (1.11–1.26) |
||||||||||
Degarelix |
5512 (16.3%) |
1190 |
21.6% (20.6–22.7%) |
1.32 (1.22–1.43) |
1.32 (1.22–1.43) |
||||||||||
Triptorelin |
1260 (3.7%) |
324 |
25.7% (23.3–28.2%) |
1.66 (1.45–1.89) |
1.72 (1.50–1.98) |
||||||||||
CI = confidence interval. * Adjusted for age, state of residence, ADT medication. | |||||||||||||||
Received 25 April 2022, accepted 13 October 2022
- Mariya F Hamid1
- Amy Hayden2,3
- Tania Moujaber2,4
- Sandra Turner2,4
- Howard Gurney2,5
- Mathis Grossmann6,7
- Peter Wong1,4
- 1 Westmead Hospital, Sydney, NSW
- 2 Crown Princess Mary Cancer Centre at Westmead Hospital, Sydney, NSW
- 3 Western Sydney University, Sydney, NSW
- 4 Westmead Clinical School, the University of Sydney, Sydney, NSW
- 5 Macquarie University, Sydney, NSW
- 6 Austin Health, Melbourne, VIC
- 7 The University of Melbourne, Melbourne, VIC
We thank Karen Byth (Research and Education Network, Western Sydney Local Health District) for assisting with the statistical analysis.
Peter Wong is a site investigator in an Amgen‐sponsored post‐marketing trial assessing medication adherence and has received speaking honoraria from Amgen. He is also Honorary Medical Director of Healthy Bones Australia.
- 1. Australian Institute of Health and Welfare; Cancer Australia. Prostate cancer in Australia statistics. Updated 15 Sept 2022. https://www.canceraustralia.gov.au/cancer‐types/prostate‐cancer/statistics (viewed Sept 2022).
- 2. Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am 2011; 40: 655‐671.
- 3. Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol 2002; 167: 1952‐1956.
- 4. Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005; 90: 6410‐6417.
- 5. Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long‐term androgen deprivation therapy in patients with prostate cancer. Urology 2007; 69: 500‐504.
- 6. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen‐deprivation therapy in men with prostate cancer. Cancer 2009; 115: 2388‐2399.
- 7. Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003; 169: 2008‐2012.
- 8. Ryan CW, Huo D, Demers LM, et al. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol 2006; 176: 972‐978.
- 9. Ryan CW, Huo D, Bylow K, et al. Suppression of bone density loss and bone turnover in patients with hormone‐sensitive prostate cancer and receiving zoledronic acid. BJU Int 2007; 100: 70‐75.
- 10. Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once‐weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med 2007; 146: 416‐424.
- 11. Greenspan SL, Nelson JB, Trump DL, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen‐deprivation therapy. J Clin Oncol 2008; 26: 4426‐4434.
- 12. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745‐755.
- 13. Grossmann M, Gilfillan C, Bolton D, et al. Bone and metabolic health in patients with non‐metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust 2011; 194: 301‐306. https://www.mja.com.au/journal/2011/194/6/bone‐and‐metabolic‐health‐patients‐non‐metastatic‐prostate‐cancer‐who‐are
- 14. Cancer Council Australia Advanced Prostate Cancer Guidelines Working Party. Clinical practice guidelines for the management of locally advanced and metastatic prostate cancer. Updated 2010. https://wiki.cancer.org.au/australia/Guidelines:%20Prostate_cancer/Management/Locally_advanced_and_metastatic (viewed Apr 2022).
- 15. National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management [Clinical guideline NG131]. Updated 15 Dec 2021. www.nice.org.uk/guidance/ng131 (viewed Apr 2022).
- 16. Mottet N, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618‐629.
- 17. Alibhai SMH, Zukotynski K, Walker‐Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone‐targeted therapies for prostate cancer: a programme in evidence‐based care. Cancer Care Ontario clinical practice guideline. Clin Oncol (R Coll Radiol) 2017; 29: 348‐355.
- 18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone‐targeted therapies for prostate cancer: ASCO endorsement of a Cancer Care Ontario guideline. J Clin Oncol 2020; 38: 1736‐1743.
- 19. Pan B, Aherne NJ, Shakespeare T, et al. Bone health assessment with dual energy X‐ray absorptiometry in men with high‐risk prostate carcinoma commencing adjuvant androgen deprivation therapy. Rep Pract Oncol Radiother 2022; 27: 677‐683.
- 20. Alibhai SM, Yun L, Cheung AM, Paszat L. Screening for osteoporosis in men receiving androgen deprivation therapy. JAMA 2012; 307: 255‐256.
- 21. Suarez‐Almazor ME, Luo R, et al. Low rates of bone mineral density measurement in Medicare beneficiaries with prostate cancer initiating androgen deprivation therapy. Support Care Cancer 2014; 22: 537‐544.
- 22. Ewald DP, Eisman JA, Ewald BD, et al. Population rates of bone densitometry use in Australia, 2001–2005, by sex and rural versus urban location. Med J Aust 2009; 190: 126‐128. https://www.mja.com.au/journal/2009/190/3/population‐rates‐bone‐densitometry‐use‐australia‐2001‐2005‐sex‐and‐rural‐versus
- 23. Australian Department of Health. National strategic action plan for osteoporosis 2019. https://www.health.gov.au/sites/default/files/documents/2020/01/national‐strategic‐action‐plan‐for‐osteoporosis‐2019_1.pdf (viewed July 2022).
- 24. Jones JM, Tsang DS, Zheng S, et al. Implementing and evaluating the impact of BoneRx: a healthy bone prescription for men with prostate cancer initiating androgen deprivation therapy. J Clin Med 2022; 11: 2703.
- 25. Ito K, Elkin EB, Girotra M, Morris MJ. Cost‐effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med 2010; 152: 621‐629.





Abstract
Objective: To determine the prevalence in Australia of bone health assessment of men with prostate cancer by dual‐energy x‐ray absorptiometry (DXA), from six months before to twelve months after initiation of androgen deprivation therapy (ADT).
Design, setting: Cross‐sectional national study; linkage of de‐identified Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) data.
Participants: Men (18 years or older) first dispensed PBS‐subsidised ADT during 1 May 2017 – 31 July 2020.
Main outcome measures: Prevalence of MBS‐subsidised DXA assessments undertaken from six months before to twelve months after first ADT prescription.
Results: Of 33 836 men with prostate cancer commencing ADT therapy during 2017–20, 6683 (19.8%) underwent DXA bone heath assessments between six months before and twelve months after commencing ADT; the mean time from first ADT dispensing to DXA scanning was +90 days (standard deviation, 134 days). The proportion of men aged 54 years or younger who had scans (66 of 639, 10%) was smaller than that of men aged 70–84 years (4528 of 19 378, 23.4%; adjusted odds ratio, 0.36; 95% CI, 0.28–0.47).
Conclusions: For about 80% of men with prostate cancer commencing ADT in Australia, therapy initiation was not accompanied by DXA assessment of bone health. Given the excellent long term prognosis for men with prostate cancer and the availability of bone protective therapy, bone health monitoring should be a routine component of prostate cancer care for men receiving ADT.