Ascites is the pathological accumulation of fluid in the peritoneal cavity.1 Cirrhosis is the commonest cause of ascites in developed countries, followed by malignancy and cardiac failure.1 Ascites is a common presenting sign of hepatic decompensation in cirrhosis, with important prognostic implications.1 Spontaneous bacterial peritonitis is bacterial infection of ascites without any obvious source of infection.1,2 It occurs in 1–4% of outpatients and 10% of inpatients with ascites secondary to cirrhosis, and can be asymptomatic initially but life‐threatening when diagnosis is delayed.1,3
Ascitic fluid analysis is recommended to evaluate the cause of clinically apparent (grade 2 and 3) ascites (Box 1) at first presentation and to exclude spontaneous bacterial peritonitis in patients with known cirrhosis, clinical deterioration (new neurological, liver or renal dysfunction) or acute gastrointestinal bleeding.1 Paracentesis (from the Greek parakentein, meaning “to pierce at the side”) is therefore a useful clinical skill to master. Large volume paracentesis can provide symptomatic relief in patients with tense ascites or pleural effusions secondary to ascites (hepatic hydrothorax).1,2,3
Definitions
Up to 30 mL of peritoneal fluid is physiologically normal (especially in menstruating females). Transabdominal ultrasound can detect ≥10 mL of fluid. Shifting abdominal dullness on examination requires about 1500 mL of fluid.1 Ascites volume is graded clinically from mild to large (Box 1).1,2 No treatment is recommended for grade 1 ascites.2 Large volume paracentesis is arbitrarily defined as drainage of > 5000 mL of ascitic fluid in a single procedure, as removal of > 5000 mL in patients with cirrhosis requires plasma expansion (usually with albumin) to avoid post paracentesis circulatory dysfunction.2 Secondary bacterial peritonitis is the rare occurrence of peritonitis in a patient with cirrhosis secondary to organ perforation or poor aseptic technique, suggested by focal abdominal pain, very high neutrophil count, and multiple organisms on ascites culture (Box 2).1
Patient preparation
Paracentesis is a low bleeding risk procedure which can be carried out safely even in patients with advanced cirrhosis.1,3,4,5,6 Guidelines recommend that coagulopathy or thrombocytopenia should not be routinely corrected, but patients with platelet count < 20 × 109/L or chronic kidney disease may be at higher risk of bleeding.4,5,6 Antibiotic prophylaxis is not required before paracentesis; neither is withholding anti‐platelet or anti‐coagulant medications.5 Published major bleeding rates following paracentesis range from 0 to 2.7% and are lower with use of ultrasound guidance.1 A drug history should be taken to guide anti‐coagulant reversal if significant bleeding occurs.
Baseline full blood count, and liver and renal function blood tests are useful to assess baseline organ function and calculate the serum albumin ascites gradient. Serum (blood) brain natriuretic peptide should be requested in an ethylenediaminetetraacetic acid (EDTA) tube if cardiac failure is suspected as the cause of ascites. Elevated levels of brain natriuretic peptide (> 300 pg/mL) may serve as a marker of disease severity and subclinical cardiac dysfunction in patients with cirrhosis.7 Ultrasound of the liver can document organomegaly, liver or peritoneal lesions, established cirrhosis, and signs of portal hypertension.
Paracentesis risks
Paracentesis requires informed patient consent, including for intravenous albumin replacement if large volume paracentesis is being performed.1,2 The most common clinically significant complications of paracentesis are fluid leak (3–13%), bleeding (0–2.7%) and organ puncture (< 1%), although routine use of ultrasound for paracentesis likely reduces these risks.1,8,9 Caution is advised in patients with clinical signs of bowel or bladder obstruction, significant central adiposity, grade 1 ascites (Box 1), loculated ascites and during pregnancy. Such patients often require paracentesis by experienced proceduralists with imaging guidance.
Equipment
Paracentesis is regularly performed at the bedside under local anaesthetic, with minimal discomfort, by a variety of health care professionals.1
Paracentesis is an aseptic procedure requiring strict skin antisepsis and sterile gloves. Diagnostic paracentesis can be performed with a long 22‐gauge needle, 20 mL syringes and an antibacterial skin cleaning solution. A disposable drape is useful. A single EDTA blood tube, three plain sterile pots and two blood culture bottles are required for ascites biochemistry, cytology and microscopy. Sending ≥ 80 mL of ascites for testing significantly increases the diagnostic yield of cytology, as does use of sterile blood culture bottles if infection is suspected.1,10 Box 2 lists the tests to request, depending on the patient's presentation and suspected cause.1,3
For large volume paracentesis, commercially available paracentesis drains are available consisting of a 15 cm plastic drainage catheter with multiple drainage holes near the tip, on a long 18‐gauge puncture needle (coaxial technique) (Box 3). Once the central needle is removed, rapid fluid drainage occurs through the catheter, which can be secured to the patient. Using an intravenous cannula for large volume paracentesis is discouraged; these are often too short and prone to kinking or dislodging.
Procedure
The patient should be positioned supine or slightly head up. Bedside ultrasound is recommended to assess the most suitable site for drainage.1 Ultrasound allows confirmation of ascites and the estimated volume, location of viscera, and the depth from the anterior abdominal wall to the fluid. Ultrasound marking of the proposed skin entry site should be performed with a permanent marker immediately before paracentesis, with the patient in the same position. Avoid access through infected skin, haematomas, peritoneal masses and scars.11 Experienced clinicians can use ultrasound guidance to advance the needle tip under continuous vision into the ascites, which allows grade 1 ascites to be targeted safely.
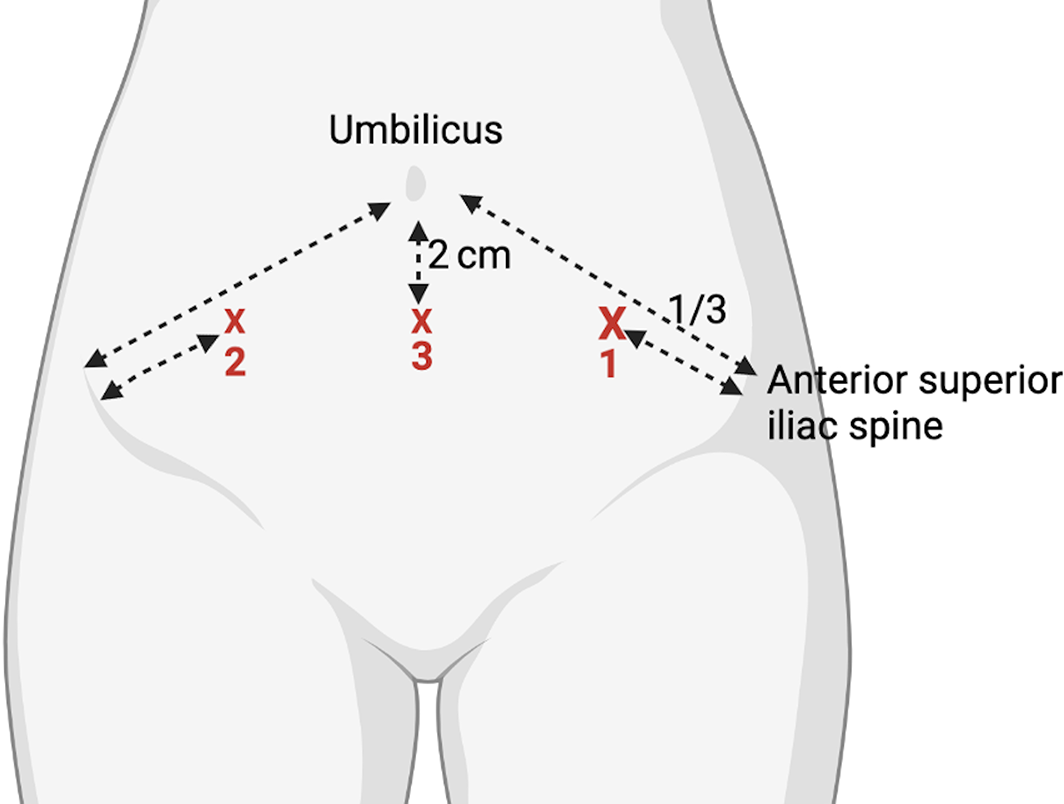
If ultrasound is not available, then a skin entry site one‐third of the way along a line drawn from the anterior iliac spine to the umbilicus in the left iliac fossa is an easily identifiable surface landmark (contralateral McBurney's point) (Box 4). The anterior abdominal wall is thinnest here; it is lateral to the inferior epigastric artery and away from the mobile caecum.11 Alternatively, the right iliac fossa or 2 cm inferior to the umbilicus can be used.11 If the infra‐umbilical puncture site is chosen, the patient should be encouraged to empty their bladder beforehand to avoid bladder injury.
The anterior abdomen needs to be widely exposed and the skin cleaned with a suitable antiseptic. The chosen entry site is anaesthetised down to the peritoneum with 5–10 mL of 1% lidocaine using a 25‐gauge needle. An oblique trajectory between the skin and the peritoneal puncture sites may reduce the chance of post paracentesis fluid leak. A small skin nick with a number 11 blade can facilitate drain passage.
Using the same trajectory as the anaesthetic needle, the paracentesis needle is advanced slowly into the peritoneal cavity while aspirating on the attached syringe, until ascites is aspirated. The free hand can steady the needle shaft to prevent sudden forward movement as the peritoneum is pierced. Often a drainage catheter is inserted; similar to intravenous cannulation, the plastic catheter is slid forward off the needle once the peritoneum is punctured and the drainage catheter is secured to the skin (coaxial technique) (Box 3). A small randomised controlled trial suggested that the z‐tract technique, where the abdominal wall skin is retracted during skin puncture and released before peritoneal puncture, is more painful for the patient, more difficult for the proceduralist, and has no difference in post paracentesis fluid leakage.9
Dispose of all sharps and write a brief procedure note in the patient's medical record, including when the drain should be removed (within 4 hours for large volume paracentesis to avoid secondary infection) and whether albumin replacement is required (routinely for large volume paracentesis of > 5000 mL in patients with cirrhosis).1,2 Radiologists often use locked pigtail drains, which have an internal suture holding the pigtail tightly in a loop after placement. The locking mechanism is visible at the catheter hub (Box 5). Locked pigtail drains require unlocking before removal to allow the pigtail to uncurl — this will be documented in the procedure note. A video of the paracentesis procedure can be reviewed along with this article to refresh knowledge before performing the procedure.12
Care after paracentesis
Routine clinical observations and puncture site checks for leakage or haematoma are required, as well as regular monitoring of drainage output. Small fluid leaks or haematomas are usually successfully treated with direct manual pressure. Haemorrhagic ascites (> 10 000 red blood cells per microlitre) is uncommon and is usually spontaneous due to advanced liver disease or secondary to hepatocellular carcinoma, rather than a complication from paracentesis.13 In patients with haemorrhagic ascites or clinical suspicion of active bleeding (expanding haematoma, acute cardiovascular instability) or suspected organ perforation (spontaneous rupture of hepatocellular carcinoma, iatrogenic injury, secondary bacterial peritonitis), a low threshold for urgent computed tomography of the abdomen with intravenous contrast is advised, followed by discussion with the local surgical team or interventional radiology.1,13
Ascitic fluid leak is the most common complication of paracentesis.1,9 Leaks can be minimised by only making a small skin nick or avoiding this entirely, using a small diameter catheter (≤ 8 Fr) and an oblique catheter insertion technique. Persistent leakage usually indicates significant residual ascites which can be treated by repeat paracentesis. Otherwise, a skin suture can be considered.1 Pressure dressings should be avoided as they rapidly soak and can damage the skin.1
Patients with cirrhosis require intravenous albumin infusion (8 g albumin/L drained) after drainage of 5000 mL of ascites, to avoid post paracentesis circulatory dysfunction. For example, 40 g albumin should be infused if 5000 mL ascites is drained.1,2,3 This is equivalent to two 100 mL bottles of 20% salt poor albumin. Limiting paracentesis to ≤ 8000 mL in a single session over 4 hours before drain removal may reduce post paracentesis circulatory dysfunction and infection.1 Patients with malignant ascites do not require routine albumin replacement and can be drained to dryness over 8 hours, often as a day case procedure.14 We do not recommend longer drainage times, as these increase protein losses and the risk of secondary infection.
Depending on the cause of the ascites, the patient will need referral to the appropriate specialist for further care.1,2,3 Patients with cirrhosis and refractory ascites can be considered for transjugular intrahepatic portosystemic shunt insertion or liver transplantation in specialised units.1,2,3 Patients with refractory malignant ascites can be considered for tunnelled drain insertion, with quality‐of‐life benefits and cost savings over repeated paracentesis.15 However, the role of tunnelled drains for refractory ascites in patients with advanced cirrhosis is unclear and should be decided on a case by case basis after multidisciplinary team discussion.15
Box 1 – International Ascites Club grading of ascites2,3
|
Grade |
Definition |
||||||||||||||
|
|
|||||||||||||||
|
1 |
Mild: only detectable on ultrasound |
||||||||||||||
|
2 |
Moderate: causing symmetrical abdominal distension |
||||||||||||||
|
3 |
Large: causing marked abdominal distension |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Tests to request on ascitic fluid samples*
|
Test requested |
Specimen bottle |
First presentation of ascites |
Recurrent ascites |
||||||||||||
|
|
|||||||||||||||
|
Cell count and differential |
EDTA (purple) blood tube |
Yes |
Yes |
||||||||||||
|
Culture |
Blood culture bottles (≥ 10 mL in each bottle) |
Yes |
Yes |
||||||||||||
|
Serum albumin‐ascitic gradient |
Sterile pot† |
Yes |
Yes |
||||||||||||
|
Protein concentration |
Sterile pot† |
Yes |
Yes |
||||||||||||
|
Glucose concentration, lactate dehydrogenase |
Sterile pot† |
Yes (if secondary bacterial peritonitis suspected) |
Yes (if secondary bacterial peritonitis suspected) |
||||||||||||
|
Cytology (CA 19‐9, CA 15‐3, CEA)‡ |
Sterile pot† (ideally > 80 mL sample) |
If malignant cause suspected |
No |
||||||||||||
|
Adenosine deaminase |
Sterile pot† |
If tuberculous ascites suspected |
No |
||||||||||||
|
Amylase |
Sterile pot† |
If pancreatic ascites suspected |
No |
||||||||||||
|
Triglycerides and chylomicrons |
Serum‐separating blood tube (yellow top) |
If chylous ascites suspected |
No |
||||||||||||
|
|
|||||||||||||||
|
EDTA = ethylenediaminetetraacetic acid. * Remember that a patient may have several co‐existing potential causes of ascites (eg, liver and cardiac disease), so determining the cause is important for the optimum treatment. † Usually three separate sterile pots are needed so that samples can be sent to separate biochemistry, cytology and microbiology laboratories. Ideally > 100 mL of fluid is required. ‡ CA‐125 has no discriminatory role in assessment of ascites causation as it is elevated with any cause. |
|||||||||||||||
Box 3 – Coaxial 8 Fr × 15 cm paracentesis safety needle and drainage catheter
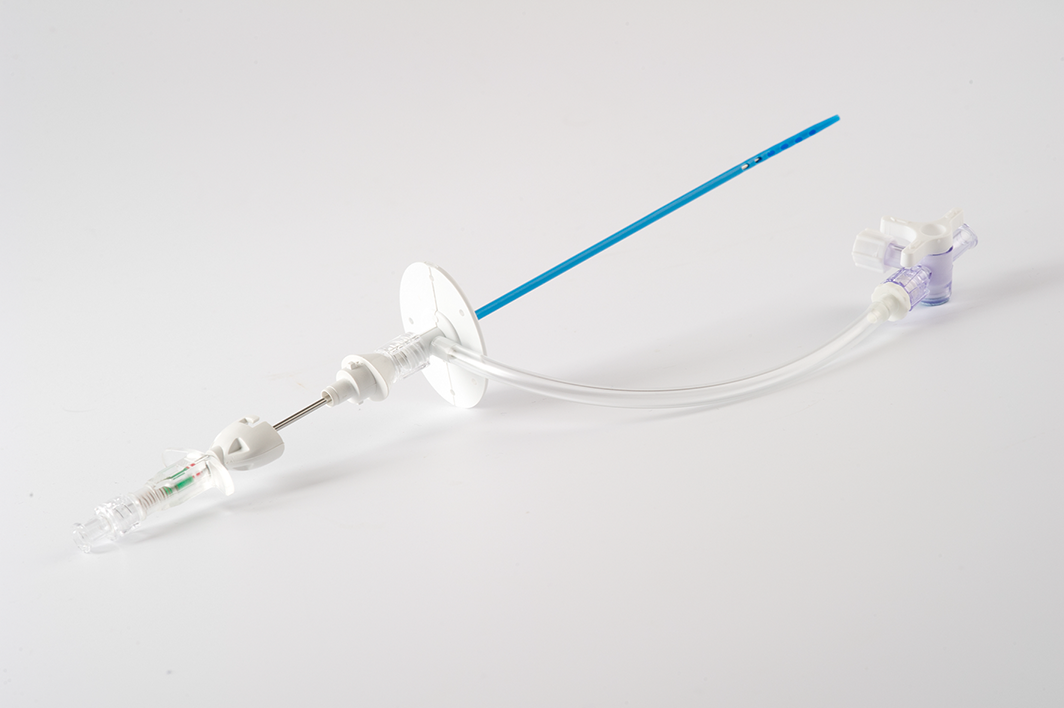
Note multiple side holes on the drainage catheter and auto‐retracting needle tip. Reproduced with the kind permission of Rocket Medical.
Box 4 – Surface markings for paracentesis puncture in order of preference (1 to 3) if ultrasound guidance is not available
Box 5 – Locking pigtail catheter containing an internal suture holding the tip in the looped position after insertion
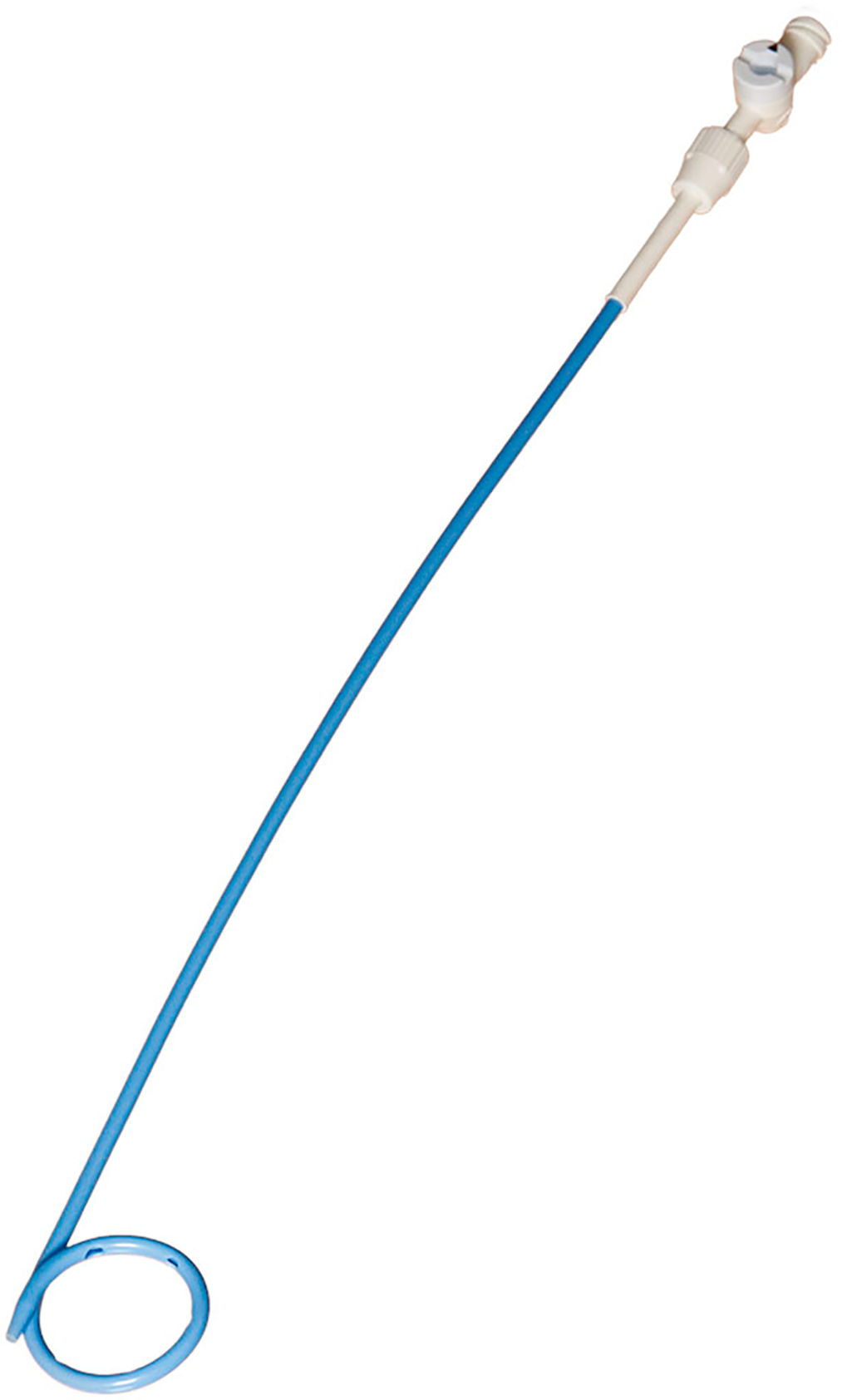
Note locking mechanism at the catheter hub, which must be undone before drain removal. Media provided courtesy of Boston Scientific. © 2022 Boston Scientific Corporation or its affiliates. All rights reserved.
Provenance: Not commissioned; externally peer reviewed.
- 1. Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut 2021; 70: 9‐29.
- 2. Biggins SW, Angeli P, Garcia‐Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021; 74: 1014‐1048.
- 3. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018; 69: 406‐460.
- 4. O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology 2019; 157: 34‐43.e1.
- 5. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image‐guided interventions – Part II: Recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol 2019; 30: 1168‐1184.e1.
- 6. Intagliata NM, Argo CK, Stine JG, et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost 2018; 118: 1491‐1506.
- 7. Araujo T, Vohra I, Palacios P, et al. B‐type natriuretic peptide (BNP) predicts 90‐day mortality and need for paracentesis in cirrhotic patients without systolic heart failure. Sci Rep 2021; 11: 1697.
- 8. Macken L, Palaniyappan N, Verma S, Aithal G. Large volume paracentesis: to do or where to do? Gut 2021; 70: 2401‐2402.
- 9. Shriver A, Rudnick S, Intagliata N, et al. A randomized controlled trial of procedural techniques for large volume paracentesis. Ann Hepatol 2017; 16: 279‐284.
- 10. Rooper LM, Ali SZ, Olson MT. A specimen volume of >/=80 mL improves cytologic sensitivity for malignant ascites: a retrospective analysis of 2665 cases. J Am Soc Cytopathol 2016; 5: 301‐305.
- 11. Siau K, Robson N, Bollipo S, Global Online Alliance for Liver Studies. Where should ascitic drains be placed? Revisiting anatomical landmarks for paracentesis. Gut 2021; 70: 2216‐2217.
- 12. Thomsen TW, Shaffer RW, White B, Setnik GS. Videos in clinical medicine Paracentesis. N Engl J Med 2006; 355: e21.
- 13. Urrunaga NH, Singal AG, Cuthbert JA, Rockey DC. Hemorrhagic ascites. Clinical presentation and outcomes in patients with cirrhosis. J Hepatol 2013; 58: 1113‐1118.
- 14. Harding V, Fenu E, Medani H, et al. Safety, cost‐effectiveness and feasibility of daycase paracentesis in the management of malignant ascites with a focus on ovarian cancer. Br J Cancer 2012; 107: 925‐930.
- 15. Macken L, Corrigan M, Prentice W, et al. Palliative long‐term abdominal drains for the management of refractory ascites due to cirrhosis: a consensus document. Frontline Gastroenterol 2022; 13: e116‐e125.






Open access
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
No relevant disclosures.