Clinical record
A 21‐year‐old man who migrated to Australia from Nepal 4 years previously was referred to a dermatologist. He had a 12‐month history of a polymorphic eruption consisting of widespread macules, plaques, papules, and nodules. As these were asymptomatic, he had not previously sought medical attention.
The patient reported a history of nasal stuffiness, pedal oedema, and malaise. He also noticed numbness in his fingers bilaterally over the previous few months, leading him to scald his hands while working as a kitchen assistant.
Examination identified shiny papules on his wrists, lateral aspects of the feet, and ear helices (Box 1, A, B and C), with no hypoaesthesia. Multiple hypopigmented and erythematous annular plaques were noted, with hypoaesthesia on his trunk (Box 1, D and E). He had well defined patches with ichthyosis, hypopigmentation and erythema on his shins bilaterally, with hypotrichosis and anaesthesia (Box 1, F).
As the patient was from a leprosy endemic region, and had sensory symptoms along with polymorphic lesions, a diagnosis of leprosy was considered. Superficial nerves commonly involved in leprosy were palpated, including the superficial branch of the radial nerve, and the ulnar, supraorbital, greater auricular, common peroneal, and posterior tibial nerves. Non‐tender, regular nerve thickening which was bilateral but asymmetric was noted. His voluntary motor testing was unremarkable.
Given the polymorphic rash, multiple biopsies at the edge of lesions were done to accurately establish the diagnosis. Biopsy samples from his ear, back and foot showed foamy histiocytes throughout the dermis with intracytoplasmic vacuoles, consistent with multibacillary leprosy (Box 2, A). A left wrist biopsy showed a granulomatous response with perineural histiocytic infiltrates, consistent with paucibacillary leprosy. Wade–Fite staining confirmed the presence of acid‐fast bacilli in the cytoplasm of histiocytes (Box 2, B), with polymerase chain reaction testing confirming presence of Mycobacterium leprae. Serological investigations and chest x‐ray for comorbid conditions and clinical mimics that can present with a polymorphic rash were negative for syphilis, human immunodeficiency virus, strongyloidiasis, tuberculosis, and sarcoidosis.
Due to the high incidence of ocular involvement in leprosy, an eye examination was performed by an ophthalmologist, which was unremarkable. The local public health unit was notified, with close contacts identified and assessed for leprosy. The patient was referred to the infectious diseases department and commenced on a multibacillary leprosy regimen consisting of rifampicin 600 mg monthly, clofazimine 300 mg monthly and 50 mg daily, and dapsone 100 mg daily, for at least 12 months.
Discussion
In 2020, the World Health Organization reported 127 558 new cases of leprosy worldwide.1 In Australia, leprosy is a notifiable disease, with 9 cases reported in the past year, and an average of 9.6 notifications annually over the past 5 years.2
Leprosy is caused by the obligate intracellular bacterium Mycobacterium leprae, which is believed to be transmitted through airborne droplets.3 Only 5–20% of people exposed to M. leprae will acquire leprosy.4 Those who have prolonged exposure to an untreated individual, are immunosuppressed, or have certain genetic polymorphisms in genes that affect immunity are at increased risk of acquiring leprosy. In New South Wales, any individual who has lived in a household‐like setting with a confirmed case of leprosy for a month is considered a contact.5
Most locally acquired cases occur in Indigenous Australians who reside in remote communities such as in the Northern Territory and Far North Queensland.6 However, leprosy may present in people who have lived in or travelled to endemic areas such as the Indian subcontinent, Brazil or Indonesia.
Leprosy primarily affects the skin and superficial nerves, with severity determined by the level of cell‐mediated immunity towards M. leprae. Individuals with an enhanced immune response develop paucibacillary leprosy, whereas those with an impaired immune response develop multibacillary leprosy. These subtypes are associated with certain clinicopathological features (Box 3).3,7,8 Patients may progress from paucibacillary to multibacillary leprosy, such that an overlap of features may be seen, as in our patient.
Leprosy has an average incubation period of 5 years but can vary between 2 and 20 years.3 Given the slow replication of M. leprae, the initial appearance of clinical features is indolent (Box 3). However, some patients present with an inflammatory reaction towards M. leprae, manifesting as fevers, malaise, inflammation within skin lesions, neuritis, or erythema nodosum‐like lesions, which require urgent intervention.
Leprosy may progress without treatment, leading to permanent functional disabilities (Box 3). To prevent this, prompt identification and treatment is essential, ideally in a multidisciplinary team setting (Supporting Information). Multidrug treatment is recommended for at least 12 months, and includes rifampicin, clofazimine, and dapsone.7 These medications are provided nationally without associated costs for patients.
Owing to the risk of reactivation and relapse, regular follow‐up is required up to 5 years after treatment completion.
- Early diagnosis and treatment of leprosy is critical to prevent irreversible deformity and disability, and to prevent community transmission.
- As the incubation period for Mycobacterium leprae can be up to 20 years, clinical manifestations may not be apparent for many years after exposure.
- Leprosy should be suspected in patients who present with skin lesions with or without sensory loss, along with neurological symptoms.
- Although leprosy is uncommon in Australia, it should be considered in vulnerable patients such as Indigenous Australians, and those with a history of residence in or travel to endemic areas.
Box 1 – Physical examination findings
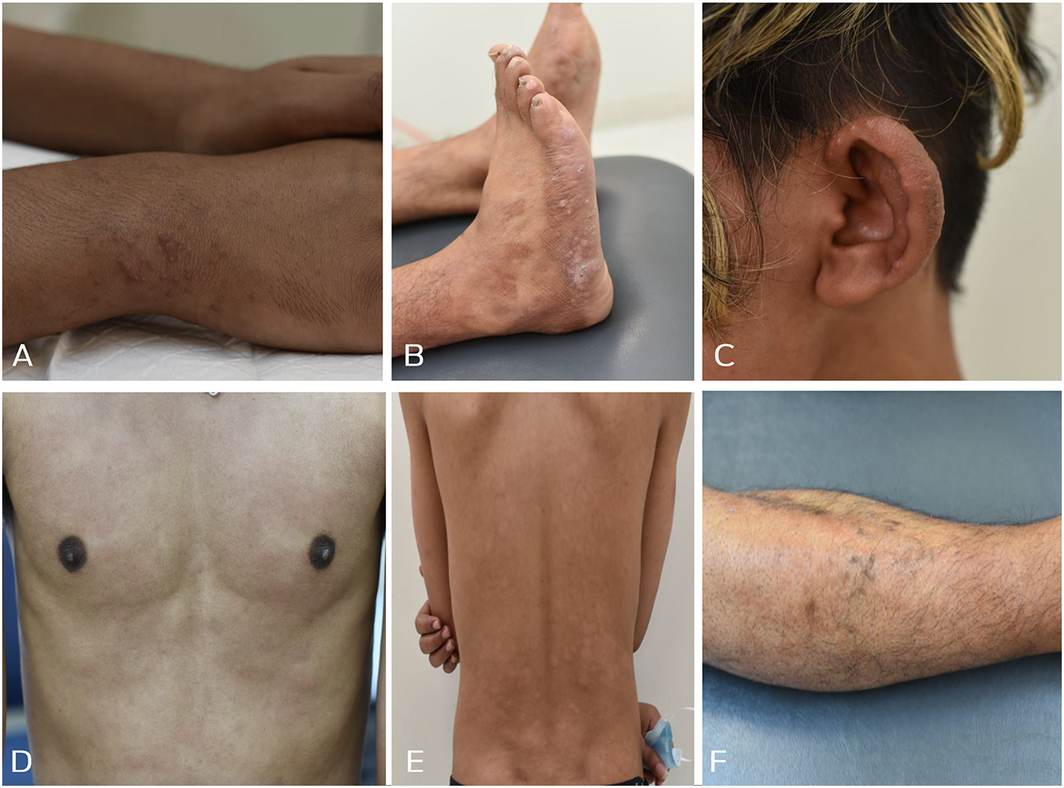
A: Multiple papules located on the patient’s wrists. B: Papules and nodules located on the lateral aspects of his feet. C: Nodules and skin thickening on his ears bilaterally. D: Multiple erythematous plaques on his torso symmetrically. E: Multiple hypopigmented macules on his back symmetrically. F: Patch of ichthyosis with hypopigmentation, erythema and hypotrichosis overlying his lower leg.
Box 2 – Histopathology and Wade–Fite stain of biopsied tissue
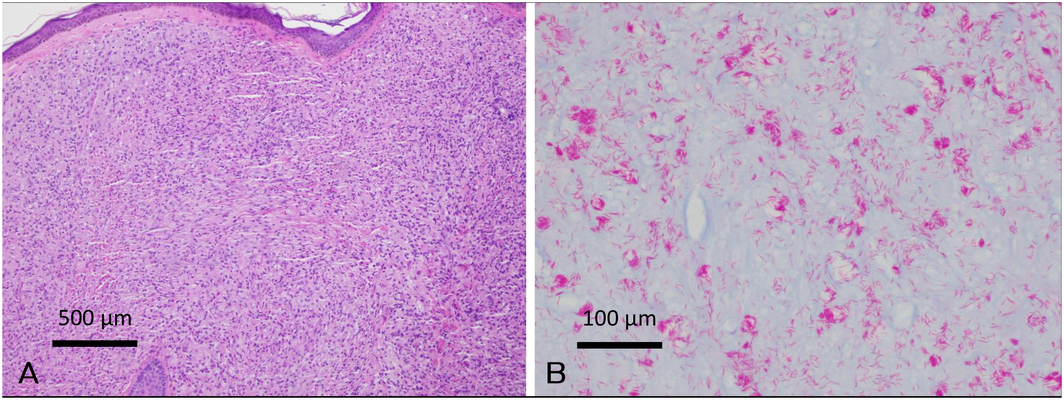
A: Sheets of histiocytes with foamy cytoplasm present throughout the dermis (haematoxylin–eosin stain, magnification × 100). B: Numerous acid‐fast bacilli within histiocytes (Wade–Fite stain, magnification × 400).
Box 3 – Clinical and histological features of paucibacillary and multibacillary leprosy3,7,8
|
|
Paucibacillary leprosy |
Multibacillary leprosy |
|||||||||||||
|
|
|||||||||||||||
|
Bacillary number |
Low |
High |
|||||||||||||
|
Number of lesions |
1–5 |
≥ 6 |
|||||||||||||
|
Histology of lesions |
Non‐caseating granulomas with giant cells |
Sheets of histiocytes with pale cytoplasm |
|||||||||||||
|
Nerve thickening |
One or few |
Multiple |
|||||||||||||
|
Sensory changes |
Occur early and are localised to lesions and major nerves |
Occur late but are extensive |
|||||||||||||
|
Distribution |
Asymmetrical |
Symmetrical |
|||||||||||||
|
Early symptoms |
Localised paraesthesia |
Nasal congestion, discharge or epistaxis |
|||||||||||||
|
Early signs |
Well defined hypopigmented or erythematous plaques |
Macules, papules, nodules or skin thickening |
|||||||||||||
|
Late symptoms |
Anaesthesia or muscle weakness within a nerve distribution (either multibacillary or paucibacillary) |
||||||||||||||
|
Late signs |
Reduced motor power and muscular atrophy, including claw hand deformity, foot drop or facial palsy (either multibacillary or paucibacillary) |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. World Health Organization. Leprosy. 2022. https://www.who.int/news‐room/fact‐sheets/detail/leprosy (viewed Jan 2022),
- 2. Australian Government Department of Health and Aged Care. National Notifiable Diseases Surveillance System (NNDSS) fortnightly reports – 11 July to 24 July 2022. https://www.health.gov.au/resources/publications/national‐notifiable‐diseases‐surveillance‐system‐nndss‐fortnightly‐reports‐11‐july‐to‐24‐july‐2022 (viewed Aug 2022).
- 3. World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. 2018. https://www.who.int/publications/i/item/9789290226383 (viewed Jan 2022).
- 4. Blok DJ, de Vlas SJ, Fischer EAJ, Richardus JH. Mathematical modelling of leprosy and its control. In: Anderson RM, Basáñez MG, editors. Advances in parasitology. Academic Press, 2015.
- 5. NSW Health. Control guidelines. Leprosy. https://www.health.nsw.gov.au/Infectious/controlguideline/Pages/leprosy.aspx (viewed Mar 2022).
- 6. Hempenstall A, Smith S, Hanson J. Leprosy in Far North Queensland: almost gone, but not to be forgotten. Med J Aust 2019; 211: 182‐183. https://www.mja.com.au/journal/2019/211/4/leprosy‐far‐north‐queensland‐almost‐gone‐not‐be‐forgotten
- 7. Northern Territory Government Department of Health. Guidelines for the control of leprosy in the Northern Territory, 2018. https://digitallibrary.health.nt.gov.au/prodjspui/bitstream/10137/526/3/Control%20of%20Leprosy%20in%20the%20Northern%20Territory%20Guidelines.pdf (viewed Mar 2022).
- 8. Government of Western Australia North Metropolitan Health Service. Guidelines for the diagnosis, management and prevention of leprosy. Feb 2019. https://ww2.health.wa.gov.au/~/media/Files/Corporate/general‐documents/Leprosy/PDF/WATBCP‐WA‐Leprosy‐Guideline.pdf (viewed March 2022).






Open access
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
We thank Timothy Gray, Staff Specialist in Microbiology and Infectious Diseases at Concord General Repatriation Hospital, for his contribution to this article.
No relevant disclosures.