The known: Clinical trials of the COVID‐19 vaccines Comirnaty and Vaxzevria have found that mild to moderate local and systemic reactions are common, but serious events are few.
The new: In the largest published post‐marketing analysis of the safety of Comirnaty and Vaxzevria, adverse events were more frequently reported by people with underlying medical conditions, including a history of anaphylaxis. Adverse event frequency was similar for Indigenous people and other Australians.
The implications: Our findings confirm the safety of Comirnaty and Vaxzevria in population use. AusVaxSafety continues to monitor COVID‐19 vaccine safety in Australia, including that of third and future doses as our vaccination program evolves.
The Australian coronavirus disease 2019 (COVID‐19) vaccination program began with Comirnaty (Pfizer–BioNTech BNT162b2) on 22 February 2021 and Vaxzevria (AstraZeneca ChAdOx1) on 9 March 2021. By 30 August 2021, more than 19 million doses of the two vaccines had been administered.1 Most people who received the vaccines during this period were both COVID‐19‐ and vaccine‐naïve, and the interval between first and second doses was varied according to evolving national vaccination policies.
As clinical trials predominantly enrol healthy people, post‐licensing monitoring is essential for assessing the safety of new vaccines. AusVaxSafety, the Australian active vaccine safety surveillance system led by the National Centre for Immunisation Research and Surveillance (NCIRS), has monitored the safety of influenza vaccines and other National Immunisation Program vaccines since 2014.2,3,4,5 On 22 February 2021, AusVaxSafety commenced surveillance of adverse events following the administration of COVID‐19 vaccines, using a survey delivered by SMS or email to people vaccinated at sentinel sites across Australia. Vaccine safety surveillance data are regularly updated on the AusVaxSafety website (www.ausvaxsafety.org.au), and detailed weekly analyses are supplied to health departments and the Therapeutic Goods Administration (TGA), including information on safety signal detection.3 Similar systems operate in the United States (v‐safe)6,7,8 and the United Kingdom (ZOE COVID Symptom Study).9
In this article, we describe the short term safety profiles of the Vaxzevria and Comirnaty vaccines (first two doses), as assessed during the first six months of COVID‐19 vaccine surveillance by AusVaxSafety.
Methods
People aged 16 years or more who received COVID‐19 vaccines during 22 February – 30 August 2021 at one of the vaccination sites monitored by AusVaxSafety were eligible for inclusion in our analysis. They were enrolled either automatically, with consent for surveillance included in their vaccination consent but with the option of opting out (state‐run vaccination hubs in New South Wales, Victoria, and Western Australia; all participating general practices; and Aboriginal Community Controlled Health Organisations [ACCHOs] in Western Australia) or by QR code self‐registration (state‐run vaccination hubs in Queensland, South Australia, Tasmania, the Australian Capital Territory, and the Northern Territory; ACCHOs other than those in Western Australia). Identical surveys were delivered by AusVaxSafety‐linked tools, including Vaxtracker,5 SmartVax,2 and the COVID‐19 Vaccine Management System.10
Three and eight days after vaccination, participants received an SMS or email message with a link to an online survey that included questions (with defined response options) regarding adverse events following immunisation (AEFI; respectively 0–3 and 4–7 days after vaccination), any medical care or advice sought (phone advice line, general practitioner or Aboriginal and Torres Strait Islander Health Worker, emergency department), impact on daily activities (“Did any of the symptoms you reported cause you to miss work, study or normal daily activities?”), and recovery (“Are you still experiencing any of the symptoms you reported?”), as well as about underlying medical conditions (Supporting Information, part 1). A reminder SMS or email was sent on days 4 and 9 if surveys were not completed. To minimise recall bias, day 3 and 8 survey responses were included only if received by days 7 or 12 respectively. In our analysis, we included responses only from people who completed both surveys. Participants were invited to complete a third survey 42 days after vaccination, but this survey is not discussed in this article.
To facilitate comparisons with overseas findings, our survey questions on AEFI included symptoms identified in clinical trials and used by v‐safe in the United States.6,7 As anaphylaxis following COVID‐19 vaccination had been reported in the United States,11 we included questions about anaphylaxis history (to medicine, vaccine, food, or other allergen). The survey also included questions about underlying medical conditions that qualified people for priority vaccination in Australia.1
Vaccination details (vaccine, batch, dose, date) and demographic details (age, sex, Indigenous status) were obtained from the vaccination record or from survey respondents directly (dose, age, sex, Indigenous status only).
Outcomes
The primary outcome was the proportion of respondents who reported any AEFI during days 0–3 after vaccination. Secondary outcomes were the proportion who reported medical review for AEFI within three days of vaccination, the proportion who reported specific adverse events, impact on daily activities, and recovery. Outcomes were compared by respondent demographic characteristics, vaccine, dose, and underlying medical conditions. The proportion of respondents who reported AEFI 0–3 days after vaccination was compared with those who reported AEFI 4–7 days after vaccination.
Statistical analysis
The proportion of participants who reported AEFI in the day 3 survey was modelled using Bayesian logistic regression. Separate models were employed for each vaccine–dose combination, and weakly informative normal prior distributions were used for model parameters. Analyses were adjusted for respondent demographic characteristics (age group, sex, Indigenous status), vaccination site type, jurisdiction, and underlying medical conditions (including anaphylaxis history). We report adjusted odds ratios (aORs) with 95% credible intervals (CrIs). All statistical analyses and modelling were conducted in R 4.1.0 (R Foundation for Statistical Computing); posterior distributions for model parameters were estimated using the RStan package (version 2.21.2). Each model was run with eight chains of 4000 iterations, and model convergence was assessed using RStan Hamiltonian Monte Carlo diagnostic tools (further details: Supporting Information, part 2).
Ethics approval
The Sydney Children’s Hospital Network Human Research Ethics Committee approved the study (HREC/16/SCHN/19).
Results
A total of 4 851 480 people received COVID‐19 vaccines at the sentinel sites during 22 February – 30 August 2021. This included about 25% of all COVID‐19 vaccine doses administered to 30 August.1 We received responses to both surveys from 3 035 983 people (overall response rate, 62.6%; Comirnaty first dose, 56.6%; second dose, 62.4%; Vaxzevria first dose, 76.6%; second dose, 79.6%). Of these respondents, 2 206 196 had been vaccinated in opt‐out hubs (72.7%), 418 828 in opt‐in hubs (13.8%), 406 247 in general practices (13.4%), 4333 in opt‐in ACCHOs (0.14%), and 379 in opt‐out ACCHOs (0.01%). The median age of Comirnaty recipients (dose 1, 42 years [interquartile range, IQR], 33–49 years; dose 21, 44 [IQR 37–49] years) was lower than that of Vaxzevria recipients (dose 1, 61 [IQR, 52–68] years; dose 2, 62 [IQR, 54–70]). A larger proportion of survey responders than of non‐responders were women (1 722 572, 56.7% v 443 251, 50.0%), and a smaller proportion were under 40 years of age (938 288, 30.9% v 1 815 130, 48.1%) (Supporting Information, table 1).
Adverse events 0–3 days after vaccination
Overall, 35.9% of respondents reported AEFI 0–3 days after Comirnaty dose 1, 54.7% after Comirnaty dose 2, 52.8% after Vaxzevria dose 1, and 22.0% after Vaxzevria dose 2 (Box 1). Local pain, fatigue, headache, and myalgia were the most frequently reported symptoms (Box 2).
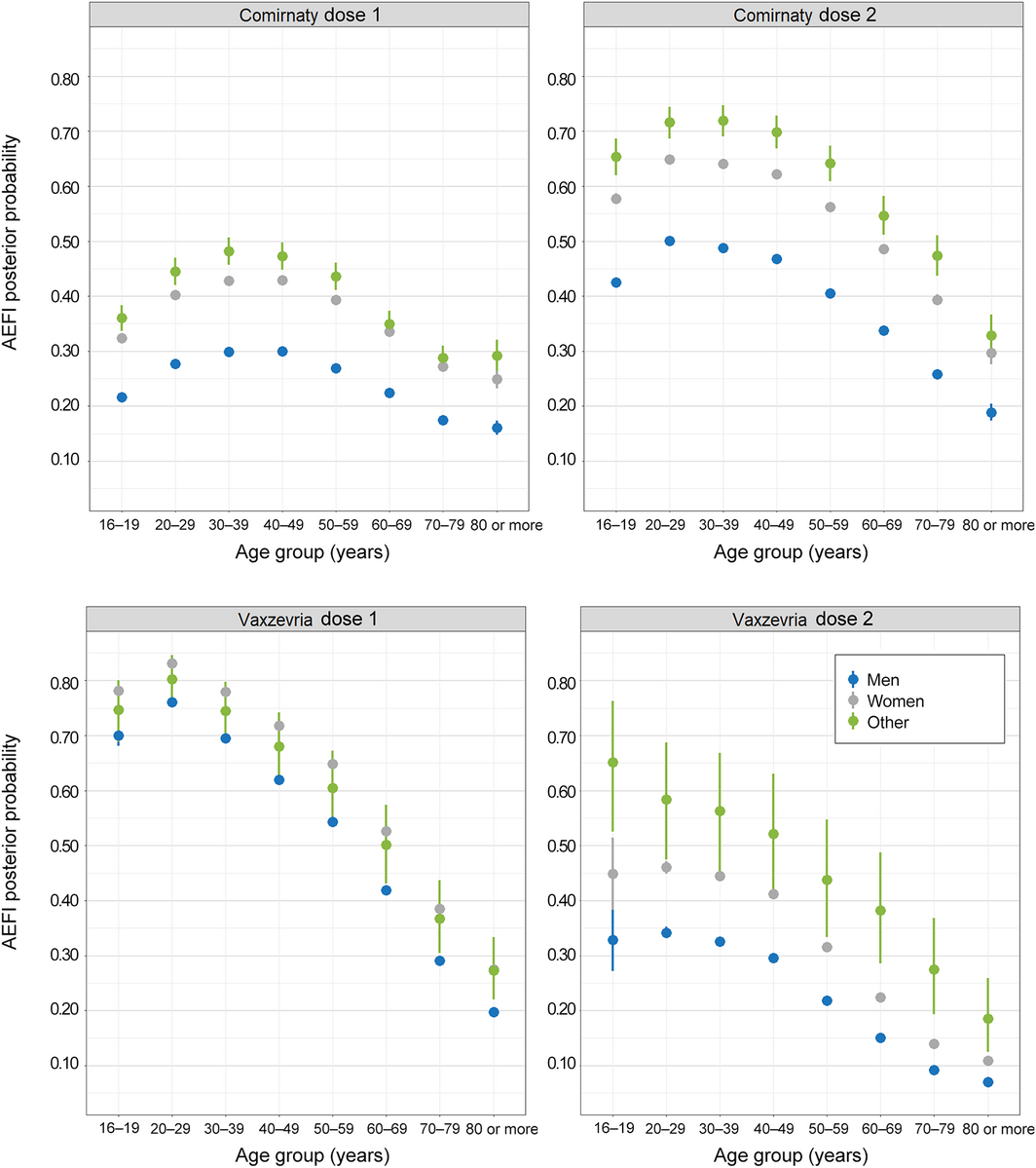
All Bayesian models converged successfully. The odds of AEFI differed by vaccination site type and jurisdiction. After adjusting for these factors, medical conditions, and demographic characteristics, the odds of reporting AEFI declined with age, except that they were lower for respondents aged 16–19 years than for those aged 20–39 years (both vaccines and both doses), and that for the first Comirnaty dose the odds rose from age 16–20 years to 30–39 years before declining. Women were more likely to report AEFI than men (both vaccines, both doses; aOR range, 1.53–1.84) (Box 3, Box 4).
Indigenous respondents were less likely than non‐Indigenous respondents to report AEFI following Comirnaty dose 2 (aOR, 0.84; 95% CrI, 0.81–0.87) or any Vaxzevria dose (dose 1: aOR, 0.71; 95% CrI, 0.67–0.75; dose 2: aOR, 0.80; 95% CrI, 0.73–0.88). Respondents with a history of anaphylaxis were more likely than those who did not to report AEFI (both vaccines, both doses; aOR range, 1.28–1.45). After adjusting for age, self‐reported medical conditions, demographic characteristics, vaccination site type, and jurisdiction, the odds of AEFI were higher for people reporting obesity (aOR range, 1.15–1.75), immunodeficiency (aOR range, 1.04–2.24), or chronic inflammatory disease (aOR range, 1.05–1.75) (Box 3).
Medical attendance and impact on daily activities
Overall, 28 298 respondents (0.9%) sought medical review in the three days following vaccination, most frequently following Comirnaty dose 2 (1.4%) or Vaxzevria dose 1 (1.2%); 5107 people (0.2%) reported emergency department presentations (Box 5).
Of those who experienced AEFI after Comirnaty dose 1, 6.8% reported that the adverse events caused them to miss work, study or routine activities, 20.3% after Comirnaty dose 2, 17.4% after Vaxzevria dose 1, and 4.2% after Vaxzevria dose 2. In each case, about 70% of people affected reported that they had missed one day or less of regular activities (Box 5).
Symptom resolution
Symptom frequency was lower 4–7 days than 0–3 days after vaccination (Box 2). Of the 1 300 162 respondents who reported AEFI during days 0–3, 996 114 reported resolution by day 3 (76.6%) and 1 126 991 (86.7%) by day 8 (Box 5).
Discussion
AusVaxSafety active post‐marketing surveillance has facilitated one of the largest reported analyses of participant‐reported COVID‐19 vaccine safety data. We report AEFI experienced within one week of vaccination with Comirnaty or Vaxzevria by more than three million respondents to our post‐vaccination survey during the first six months of the Australian adult vaccination program. The program included frontline workers from February 2021, older adults, people with underlying medical conditions, and Indigenous Australians from March 2021, and the general population from July 2021 (Comirnaty, people aged 16 years or more; Vaxzevria, people aged 18 years or more). The age distribution of recipients by vaccine reflects age‐related recommendations by the Australian Technical Advisory Group on Immunisation (ATAGI), as modified by emerging safety signals, local outbreaks, and vaccine availability.12,13
Our findings for the two COVID‐19 vaccines — 35.9% of respondents reported AEFI after Comirnaty dose 1 and 54.7% after dose 2; 52.8% after Vaxzevria dose 1, and 22.0% after dose 2 — were broadly consistent with safety profiles based on clinical trials and other post‐marketing surveillance; in phase 3 clinical trials, 27% of participants reported adverse events after receiving Comirnaty14 and 40.6% after Vaxzevria.15 The frequency rates of adverse events in our study were higher than for other vaccines used in Australia,4,5 perhaps because mRNA and viral vector vaccines more often elicit transient mild to moderate side effects than other vaccine types.16
We found that AEFI were more frequent after Vaxzevria than Comirnaty first doses, and less frequent after Vaxzevria than Comirnaty second doses. The rates of local reactions reported in our study were similar to those reported in the United Kingdom ZOE COVID Vaccine Symptom Study (29.2% and 34.2% reporting local pain following Comirnaty dose 1 and 2).9 The American v‐safe program similarly found that mRNA vaccine second doses were more reactogenic than first doses.17
As also found in the United Kingdom,9 a larger proportion of female than of male AusVaxSafety respondents reported AEFI. The odds of reporting AEFI generally declined with age, but respondents aged 16–19 years were less likely to report AEFI following Comirnaty than those aged 20–49 years. This is encouraging as vaccination programs are extended to younger adolescents.
Only 0.9% of respondents reported seeking medical review for AEFI, and only 0.2% attended emergency departments. Following influenza vaccination, 0.3% of people seek medical review (not including phone advice),5 and 1.7% following diphtheria/tetanus/pertussis/polio booster vaccination of 4‐year‐old children.18 Given the lack of data on medical review outcomes, including other reasons for seeking medical attention, these figures should be compared with caution.
While our sentinel surveillance sites included ACCHOs and other centres where Indigenous Australians are vaccinated, only 1.3% of respondents identified as Indigenous people, lower than the Australian population proportion (3%).19 This may reflect lower coverage of clinics serving Indigenous people, limited survey access, lower survey participation, or lower vaccination uptake. By 14 October 2021, only 57% of Indigenous Australians aged 16 years or more had received the first dose of a COVID‐19 vaccine and 42% the second dose, compared with 83% and 64% for all Australians.20 However, adjusted odds of AEFI for Indigenous Australians were similar to or lower than those for other Australians. We regularly forward our surveillance findings to relevant peak bodies, including the National Aboriginal Community Controlled Health Organisation, to assist with targeted safety messages for improving COVID‐19 vaccination coverage among Indigenous Australians.
In contrast to the UK ZOE COVID Symptom Study, which considered only the presence or absence of underlying medical conditions,9 AusVaxSafety provides information on AEFI in people with specific medical conditions. We found that that AEFI were more frequently reported by respondents with a history of anaphylaxis or certain medical conditions, including obesity, immunodeficiency, and chronic inflammatory disease. As participants’ reports were not validated, apparent associations may reflect confounding by a propensity to report rather than genuine relationships, and it was beyond the scope of our analysis to explore this question further. While the odds of reporting any AEFI were higher for people reporting medical conditions, the proportions of people with specific medical conditions who reported AEFI were not higher than the proportions of people aged 20–29 years who reported AEFI after vaccination (Supporting Information, table 2). Our surveillance findings can inform post‐vaccination expectations, and are being used to tailor messages to people with underlying medical conditions.
Most reported AEFI were short‐lived, and a lower proportion of respondents reported AEFI in the day 8 surveys than in the day 3 surveys (Box 2). An impact of symptoms on daily activities was most frequently reported after the second Comirnaty dose and the first Vaxzevria dose, paralleling the reporting of AEFI, and for more than half those respondents reporting an impact it lasted for no more than one day.
We supplied estimates of AEFI rates to the Therapeutic Goods Administration, the Department of Health and jurisdictional health departments, updated daily during the first two months of surveillance and weekly thereafter, and watched for abrupt increases that could indicate batch‐related (manufacturing) faults or problems related to the stepwise expansion of the vaccination program to new groups. For this reason, we took a Bayesian approach to analysis, focusing on comparing the number of new AEFI during each analysis time interval with the number predicted by previously accumulated data, and on the demographic and other characteristics of respondents in each interval. This approach took into account the changing demographic profile of vaccinated people at different stages of the vaccination program.
Limitations
The information reported by survey respondents was not clinically verified. People experiencing AEFI may be more motivated to respond, inflating the apparent frequency of AEFI. Conversely, those with severe AEFI may be less able to respond. AusVaxSafety captures only events from within a few days of vaccination. While free text reports of “other adverse events” were reviewed by a clinician, none were consistent with thrombosis with thrombocytopenia syndrome; this suggests a surveillance limitation with regard to identifying rare or AEFI of later onset. Respondents from linguistically diverse communities may have been under‐represented, as the survey was available only in English. Similarly, the representativeness of the respondents may have been limited by the need for internet access to complete the survey.
Our statistical models were designed for prediction rather than causal inference, but our adjusted odds ratios permit limited causal interpretations. For example, adjusted odds ratios by age can be interpreted as reflecting the direct effect of age on AEFI reporting, not mediated by the age‐related increase in prevalence of other medical conditions. Similarly, adjusted odds ratios by underlying condition may cautiously be interpreted as quantifying the effect of the condition, distinct from the effects of age, other medical conditions, and other factors. As vaccine doses for individual participants were not linked, we could not assess within‐individual correlations and the effects of dose interval and mixed schedules on AEFI frequency.
Conclusion
AusVaxSafety surveillance improves our understanding of the COVID‐19 vaccine safety at the population level, including in people with underlying medical conditions. As an active surveillance system, AusVaxSafety complements spontaneous AEFI reporting to the TGA,21 and helps educate and counsel people about expected AEFI and their impact during the Australian COVID‐19 vaccination program. AusVaxSafety continues to monitor COVID‐19 vaccine safety in Australia, including that of booster and future doses as our vaccination program evolves.
Box 1 – Any adverse event following COVID‐19 vaccination, as reported in the AusVaxSafety day 3 COVID‐19 vaccine safety survey, 22 February – 30 August 2021, by vaccine, dose number, and selected respondent characteristics
|
Characteristic |
Comirnaty |
Vaxzevria |
|||||||||||||
|
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
||||||||||||
|
|
|||||||||||||||
|
Any adverse event reported |
483 003/1 346 308 |
521 748/953 704 |
228 685/433 427 |
66 726/302 544 |
|||||||||||
|
Sex |
|
|
|
|
|||||||||||
|
Men |
160 764/565 158 |
181 950/399 392 |
93 652/199 643 |
23 052/136 689 |
|||||||||||
|
Women |
320 712/777 187 |
338 101/551 535 |
133 113/230 019 |
43 174/163 831 |
|||||||||||
|
Other |
782/1700 (46%) |
690/1021 (68%) |
199/285 (70%) |
40/92 (44%) |
|||||||||||
|
Age (years), median (IQR) |
42 (33–49) |
44 (37–49) |
61 (52–68) |
62 (54–70) |
|||||||||||
|
Indigenous status |
|
|
|
|
|||||||||||
|
Indigenous |
7443/20 245 |
6447/12 228 |
2230/4 551 |
625/3019 |
|||||||||||
|
Non‐Indigenous |
467 856/1 303 080 |
498 268/910 202 |
219 938/416 314 |
62 624/284 443 |
|||||||||||
|
Anaphylaxis history |
|
|
|
|
|||||||||||
|
No |
468 196/1 314 436 |
507 770/932 392 |
222 335/422 990 |
64 637/295 962 |
|||||||||||
|
Yes |
14 807/31 872 |
13 978/21 312 |
6350/10 437 |
2089/6582 |
|||||||||||
|
Underlying medical condition |
|
|
|
|
|||||||||||
|
None |
417 140/1 202 780 |
457 386/850 183 |
188 118/352 539 |
52 302/244 003 |
|||||||||||
|
Any |
65 863/143 528 |
64 362/103 521 |
40 567/80 888 |
14 424/58 541 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range.
* Complete data for all characteristics are included in the Supporting Information, table 2. |
|||||||||||||||
Box 2 – Local (at or around injection site) and other reported adverse events 0–3 (day 3 survey) or 4–7 days (day 8 survey) after vaccination with Comirnaty or Vaxzevria: AusVaxSafety COVID‐19 vaccine safety survey, 22 February – 30 August 2021*
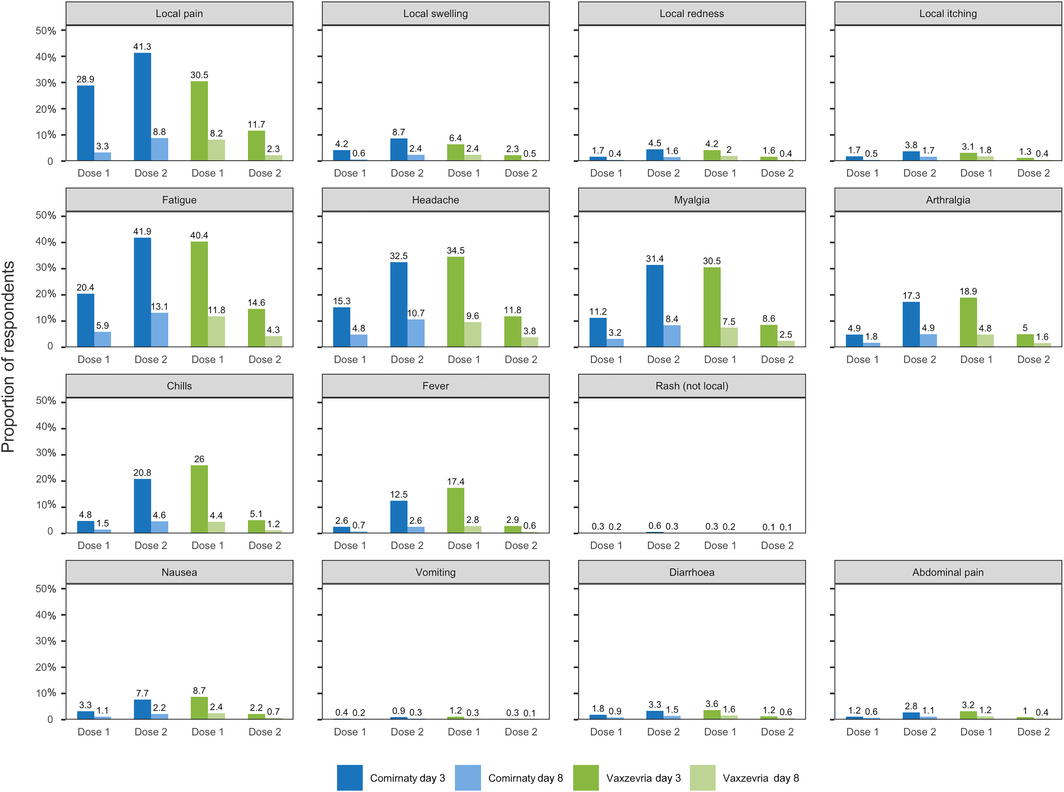
* Not shown in figure: fainting/loss of consciousness (Comirnaty dose 1/day 3: 0.12%; dose 1/day 8: 0.02%; dose 2/day 3: 0.21%; dose 2/day 8: 0.07%; Vaxzevria dose 1/day 3: 0.17%; dose 1/day 8: 0.04%; dose 2/day 3: 0.03%; dose 2/day 8: 0.01%); possible seizure (Comirnaty dose 1/day 3: 0.01%; dose 1/day 8: < 0.01%; Comirnaty dose 2/day 3: 0.02%; dose 2/day 8: 0.01%; Vaxzevria dose 1/day 3: 0.01%; dose 1/day 8: < 0.01%; Vaxzevria dose 2/day 3: < 0.01%; dose 2/day 8: < 0.01%).
Box 3 – Any adverse event 0–3 days after vaccination with Comirnaty or Vaxzevria: adjusted odds ratios by vaccine, dose, and respondent characteristics*
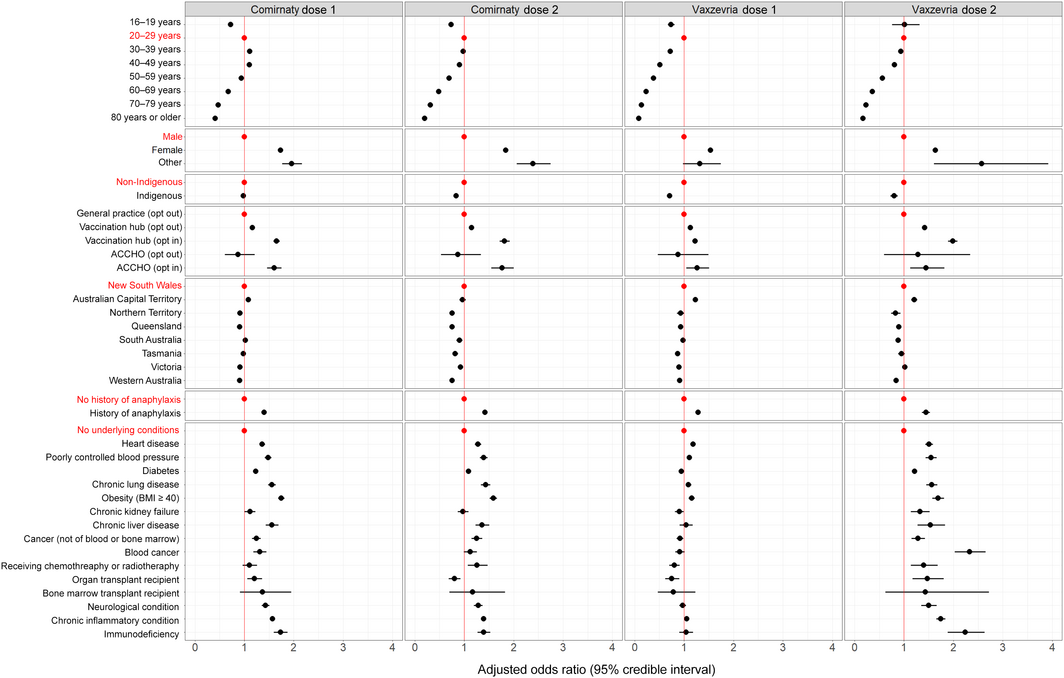
* Odds ratios are adjusted for demographic characteristics, history of anaphylaxis, and underlying medical conditions, and for vaccination site type and jurisdiction. The full data for this graph are included in the Supporting Information, table 2.
Box 4 – Any adverse event 0–3 days after vaccination with Comirnaty or Vaxzevria: mean posterior probability (with 95% credible intervals), by dose, age group, and sex
Box 5 – Any adverse event 0–3 days after vaccination with Comirnaty or Vaxzevria, medical review, impact on daily activities, and symptom resolution, by vaccine and dose
|
Outcome |
Comirnaty |
Vaxzevria |
|||||||||||||
|
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
||||||||||||
|
|
|||||||||||||||
|
Any adverse event |
483 003/1 346 308 |
521 748/953 704 |
228 685/433 427 |
66 726/302 544 |
|||||||||||
|
Adverse event/medical review sought |
8699/1 346 308 |
13 073/953 704 |
5260/433 427 |
1266/302 544 |
|||||||||||
|
Highest level medical review obtained |
|
|
|
|
|||||||||||
|
Phone advice line |
1821/8699 |
2551/13 073 |
1502/5260 |
252/1266 |
|||||||||||
|
General practice or Aboriginal and Torres Strait Islander Health Worker |
5151/8699 |
8520/13 073 |
2628/5260 |
766/1266 |
|||||||||||
|
Emergency department |
1727/8699 |
2002/13 073 |
1130/5260 |
248/1266 |
|||||||||||
|
Missed work, study or routine activities |
91 386/1 346 308 |
194 004/953 704 |
75 277/433 427 |
12 665/302 544 |
|||||||||||
|
Less than one day |
18 608/91 386 |
33 238/194 004 |
11 612/75 277 |
2286/12 665 |
|||||||||||
|
One day |
45 435/91 386 |
103 167/194 004 |
41 136/75 277 |
6727/12 665 |
|||||||||||
|
Two days |
21 288/91 386 |
47 068/194 004 |
18 478/75 277 |
2832/12 665 |
|||||||||||
|
Three or more days |
6055/91 386 |
10 531/194 004 |
4051/75 277 |
820/12 665 |
|||||||||||
|
Symptom resolution by day 3* |
396 415/483 003 |
386 138/521 748 |
163 742/228 685 |
49 818/66 726 |
|||||||||||
|
Symptom resolution by day 8* |
424 656/483 003 |
448 526/521 748 |
195 520/228 685 |
58 289/66 726 |
|||||||||||
|
|
|||||||||||||||
|
* Denominator is the number of respondents who reported any adverse event in their Day 3 survey. |
|||||||||||||||
Received 20 November 2021, accepted 10 May 2022
- Lucy Deng1,2,3
- Catherine Glover1
- Michael Dymock4,5
- Alexis Pillsbury1,2,3
- Julie A Marsh4,5
- Helen E Quinn1,2,3
- Alan Leeb6,7
- Patrick Cashman8
- Thomas L Snelling2,3
- Nicholas Wood1,2,3
- Kristine Macartney1,2,3
- 1 National Centre for Immunisation Research and Surveillance, Sydney, NSW
- 2 The Children’s Hospital at Westmead, Sydney, NSW
- 3 The University of Sydney, Sydney, NSW
- 4 Perth Children's Hospital, Perth, WA
- 5 Telethon Kids Institute, Perth, WA
- 6 SmartVax, Perth, WA
- 7 Illawarra Medical Centre, Perth, WA
- 8 Hunter New, England, Health, Newcastle, NSW
Open Access
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
AusVaxSafety surveillance is funded under a contract with the Australian Department of Health. Thomas Snelling and Nicholas Wood are supported by Career Development Fellowships from the National Health and Medical Research Council. We acknowledge the participants and staff at the surveillance sites, state and territory health departments, and the contribution of the surveillance tools SmartVax, Vaxtracker, and Microsoft COVID Vaccine Management System.
No relevant disclosures.
- 1. Australian Department of Health. COVID‐19 vaccine roll‐out. 31 Aug 2021. https://www.health.gov.au/sites/default/files/documents/2021/08/covid‐19‐vaccine‐rollout‐update‐31‐august‐2021.pdf (viewed Sept 2021).
- 2. Glover C, Crawford N, Leeb A, et al. Active SMS‐based surveillance of adverse events following immunisation with influenza and pertussis‐containing vaccines in Australian pregnant women using AusVaxSafety. Vaccine 2020; 38: 4892‐4900.
- 3. Jacoby P, Glover C, Damon C, et al. Timeliness of signal detection for adverse events following influenza vaccination in young children: a simulation case study. BMJ Open 2020; 10: e031851.
- 4. Phillips A, Glover C, Leeb A, et al. Safety of live attenuated herpes zoster vaccine in Australian adults 70–79 years of age: an observational study using active surveillance. BMJ Open 2021; 11: e043880.
- 5. Pillsbury AJ, Glover C, Jacoby P, et al. Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia. BMJ Open 2018; 8: e023263.
- 6. Gee J, Marquez P, Su J, et al. First month of COVID‐19 vaccine safety monitoring: United States, December 14, 2020 – January 13, 2021. MMWR Morb Mort Wkly Rep 2021; 70: 283‐288.
- 7. Chen G, Li X, Sun M, et al. COVID‐19 mRNA Vaccines are generally safe in the short term: a vaccine vigilance real‐world study says. Front Immunol 2021; 12: 669010.
- 8. Shimabukuro TT, Kim SY, Myers TR, et al; CDC v‐safe COVID‐19 Pregnancy Registry Team. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons. N Engl J Med 2021; 384: 2273‐2282.
- 9. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939‐949.
- 10. Department of Health (Victoria). Victorian COVID‐19 vaccination program. COVID‐19 Vaccination Management System operating model and process considerations for health services. 19 Feb 2021. https://coronavirus.wh.org.au/wp‐content/uploads/2021/03/C‐CVMS‐operating‐model‐and‐process‐considerations‐for‐health‐services‐19.02.2021.pdf (viewed May 2022).
- 11. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID‐19 vaccines in the US: December 14, 2020 – January 18, 2021. JAMA 2021; 325: 1101‐1102.
- 12. Australian Technical Advisory Group on Immunisation. ATAGI statement on revised recommendations on the use of COVID‐19 Vaccine AstraZeneca. 17 June 2021. https://www.health.gov.au/news/atagi‐statement‐on‐revised‐recommendations‐on‐the‐use‐of‐covid‐19‐vaccine‐astrazeneca‐17‐june‐2021 (viewed May 2022).
- 13. Australian Technical Advisory Group on Immunisation. ATAGI statement on use of COVID‐19 vaccines in an outbreak setting. 13 July 2021. https://www.health.gov.au/news/atagi‐statement‐on‐use‐of‐covid‐19‐vaccines‐in‐an‐outbreak‐setting (viewed May 2022).
- 14. Polack FP, Thomas SJ, Kitchin N, et al; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603‐2615.
- 15. Falsey AR, Sobieszczyk ME, Hirsch I, et al; AstraZeneca AZD1222 Clinical Study Group. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV‐19) Covid‐19 vaccine. N Engl J Med 2021; 385: 2348‐2360.
- 16. Siegrist CA. Autoimmune diseases after adolescent or adult immunization: what should we expect? CMAJ 2007; 177: 1352‐1354.
- 17. Chapin‐Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA‐based COVID‐19 vaccines. JAMA 2021; 325: 2201‐2202.
- 18. Australian Department of Health. Vaccine safety in Australia: AusVaxSafety summary report 2020. 15 Oct 2021. https://www.health.gov.au/resources/publications/vaccine‐safety‐in‐australia‐ausvaxsafety‐summary‐report‐2020 (viewed Nov 2021).
- 19. Australian Bureau of Statistics. Estimates and projections, Aboriginal and Torres Strait Islander Australians. Reference period 2006 to 2031. 11 July 2019. https://www.abs.gov.au/statistics/people/aboriginal‐and‐torres‐strait‐islander‐peoples/estimates‐and‐projections‐aboriginal‐and‐torres‐strait‐islander‐australians/latest‐release (viewed Sept 2021).
- 20. Australian Department of Health. COVID‐19 vaccination. Geographic vaccination rates: SA4 Indigenous population, 13 October 2021. https://www.health.gov.au/resources/publications/covid‐19‐vaccination‐geographic‐vaccination‐rates‐sa4‐indigenous‐population‐13‐october‐2021 (viewed May 2022).
- 21. Phillips A, Carlson S, Danchin M, et al. From program suspension to the pandemic: a qualitative examination of Australia’s vaccine pharmacovigilance system over 10 years. Vaccine 2021; 39: 5968‐5981.






Abstract
Objective: To assess the short term safety of the COVID‐19 vaccines Comirnaty (Pfizer–BioNTech BNT162b2) and Vaxzevria (AstraZeneca ChAdOx1) in Australia.
Design: Prospective observational cohort study; online surveys by AusVaxSafety, a national active vaccine safety surveillance system, three and eight days after vaccination.
Setting, participants: People aged 16 years or more who received COVID‐19 vaccines at sentinel vaccination hubs, general practices, or Aboriginal Community Controlled Health Organisation clinics, 22 February – 30 August 2021.
Main outcome measures: Primary outcome: proportion of respondents who reported any adverse event following immunisation (AEFI) 0–3 days after vaccination. Secondary outcomes: proportions of respondents who reported specific adverse events or medical review for AEFI within seven days of vaccination; impact on usual daily activities; recovery.
Results: 4 851 480 people received COVID‐19 vaccines at participating sentinel sites during the study period (25% of all COVID‐19 vaccine doses administered in Australia to 30 August 2021). 3 035 983 people responded to both surveys (response rate, 62.6%); 35.9% of respondents reported one or more AEFI 0–3 days after Comirnaty dose 1, 54.7% after Comirnaty dose 2, 52.8% after Vaxzevria dose 1, and 22.0% after Vaxzevria dose 2. Local pain, fatigue, headache, and myalgia were the most frequently reported symptoms. After adjusting for demographic characteristics, vaccination site type, jurisdiction, and self‐reported medical conditions, the odds of reporting any AEFI were higher for women than men (range of adjusted odd ratios [aORs], by vaccine and dose, 1.53–1.84), for people with a history of anaphylaxis (aOR range, 1.28–1.45), and for people reporting certain underlying conditions, including obesity (aOR range, 1.15–1.75), immunodeficiency (aOR range, 1.04–2.24), or chronic inflammatory disease (aOR range, 1.05–1.75). 0.9% of respondents sought medical advice in the three days following vaccination, most frequently after Comirnaty dose 2 (1.4%) and Vaxzevria dose 1 (1.2%).
Conclusion: AusVaxSafety active surveillance affirms the short term safety profile of Comirnaty and Vaxzevria vaccines in a large population sample during the first six months of the Australian COVID‐19 vaccination program.