People who inject drugs (PWID) are a cohort at risk of invasive infections requiring hospitalisation. These infections include abscesses, bloodstream infections, infective endocarditis and bone and joint infections. There has been a significant rise in the prevalence of invasive infections related to injecting drug use (IDU) in the past 10 years.1,2 Treatment of these infections can be complex, as it frequently requires prolonged antimicrobial therapy, management of substance use, mental health disorders, and social comorbidities, and can be complicated by challenging therapeutic relationships and stigma. Due to the spectrum of these infections and their presentations, PWID can be managed by many different clinical units in hospital, including medical, surgical and psychiatry. In this narrative review, we summarise current evidence on management strategies and highlight the priorities of care for PWID with invasive infections. This includes using a patient‐centred multidisciplinary approach to engage and support PWID, individualised antimicrobial plans, and using the hospital admission as a time to address preventive strategies to decrease future risk of morbidity and mortality (Box 1).
Methodology
We searched Ovid MEDLINE and EMBASE, PubMed and Google Scholar through to April 2022 for clinical trials, qualitative articles, reviews and clinical guidelines regarding to the care of invasive infections in PWID. Examples of search terms used include “injection drug use”, “people who inject drugs”, “endocarditis”, “osteomyelitis”, “skin and soft tissue infection”, “outpatient parenteral antimicrobial therapy”, “dalbavancin”, “oritavancin”, “addiction medicine”, “opiate substitution treatment” and “stigma”. We also manually searched the reference lists of identified articles for other relevant articles.
Engaging people who inject drugs in care
Acknowledging and addressing stigma
PWID frequently experience profound stigma from health care staff, and this significantly affects their engagement in care.3 Negative attitudes towards PWID by health care staff can be the result of perceived risk of medication misuse, behavioural challenges, and poor motivation among PWID, combined with the perception that they themselves have had inadequate training to work with PWID.3 Stigma towards PWID is associated with poorer health outcomes, including injecting‐related harms and overdose, as well as delayed presentation to health care services and increased risk of unplanned discharges.3,4
Strategies to decrease stigma include educating health care providers regarding the importance of open and empathetic communication, respecting patient autonomy and improving clinician understanding of substance use disorders and harm reduction strategies. Education provided to clinicians on identifying key moments of infection prevention in injecting drug use was found to improve clinician comfort educating PWID.5 Using a collaborative approach through multidisciplinary teams with peer support workers, if possible, acknowledging pre‐existing bias and offering patient‐centred management plans can also help reduce stigma.6 Peer support workers for PWID are staff members who have a lived experience of a substance use disorder and can provide a unique level of support which is patient‐centred.6 Peer support workers have been demonstrated to increase retention in care, improve access to opioid agonist therapy (OAT), improve communication between PWID and health care workers, provide patient advocacy, and they can help coordinate care after discharge.6,7
Open discussions regarding substance use
Addiction is a chronic, relapsing‐remitting disease that needs to be addressed to facilitate appropriate management of the invasive infection with which the patient has presented. Discussing substance use with patients in a non‐judgmental manner can also help reduce stigma and improve engagement. The key components of a drug history are summarised in Box 2. This history not only forms part of the ongoing care plan for the patient, but also helps with a risk assessment of withdrawal. It is during the initial withdrawal phase when patients are at a heightened risk of using other substances to mitigate symptoms of withdrawal, as well as an increased risk of discharge in an unplanned manner. Understanding the patient’s substance use is also essential to ensure appropriate pain management can be provided. This is particularly important as many invasive infections are complicated by acute pain. However, PWID often describe barriers to the receipt of adequate pain management, including clinician concern about “drug‐seeking” behaviour.4,8 Early referral to addiction medicine and pain services can help provide adequate analgesia to PWID which, apart from being an important therapeutic goal, is also a prerequisite for establishing a constructive therapeutic relationship.
Provision of evidence‐based treatment for people who inject drugs with invasive infections
Antimicrobial therapy and delivery
Traditionally, invasive infections are treated with 2–6 weeks of intravenous antimicrobials.9,10,11,12 Given injecting drug use is often a barrier to outpatient parenteral antibiotic programs, PWID with invasive infections frequently remain in hospital for prolonged periods of time.13 However, there is emerging evidence that support strategies beyond traditional inpatient‐based treatment for PWID with invasive infections. Here, we present the current evidence for these alternative approaches, which support the case for individual patient‐centred decision making. It is beyond the scope of this review to discuss individual antimicrobial recommendations and, instead, we focus on the delivery of these antimicrobials.
Outpatient parenteral antimicrobial therapy
Outpatient parenteral antimicrobial therapy (OPAT) facilitates the delivery of intravenous antimicrobials to patients at home or in an OPAT clinic, permitting patients to return home rather than remain in hospital. Advantages of OPAT include greater patient satisfaction with care, the ability to return to usual daily activities including work, and reduced hospital length of stay, with associated cost savings for the health care institution.14,15 There has traditionally been a resistance to enrolling PWID onto OPAT due to concerns about non‐adherence, staff safety, and tampering with central lines required for antimicrobial administration.13 There are also no guidelines regarding the admission of PWID to OPAT, with the Infectious Diseases Society of America OPAT guidelines stating that “no recommendation can be made”.14 However, there is increasing evidence that PWID can be safely discharged on OPAT.
A literature review of ten studies assessing OPAT efficacy and safety among PWID which included a total of 800 individuals found completion rates of antimicrobials for PWID receiving OPAT ranged from 72% to 100%, comparable to completion rates in other patients.15,16 Rates of central line tampering are low in available studies (0–2%),17,18,19 and no significant difference in rates of line infections have been noted.20,21 While PWID may require more intensive support when receiving OPAT (including increased after‐hour nursing calls),21 staff safety has been demonstrated.22
Intermittent injecting drug use should not be used as a definitive dismissal criterion from OPAT, with studies demonstrating OPAT success with patients who have continued to inject.18,23 Instead, admission criteria onto OPAT should be individualised and consider patient clinical stability and the availability of an appropriate antimicrobial management plan that is able to be delivered via OPAT. Social instability leading to a decreased ability to return for treatment should be considered. The authors of a 2017 study found that 41 of 67 PWID (61%) enrolled in OPAT failed treatment when single missed antibiotic doses or clinic appointments were defined as failure of OPAT treatment.17 However, this should be balanced with the risk of complete disengagement from inpatient treatment compared with potential longer term engagement with an outpatient model and individual supports through a multidisciplinary team.
Oral antimicrobials for serious infections
There has recently been a reinvigorated debate regarding the use of early oral antimicrobials for invasive infections traditionally managed with prolonged intravenous therapy.24 Two large randomised clinical trials published in 2019 provided evidence regarding the efficacy and safety of early oral antibiotic therapy to complete treatment for infective endocarditis and bone and joint infections.25,26 However, these trials are difficult to extrapolate to PWID, as PWID were either not included or were only 1% of the oral treatment arm.25,26 Data on oral antibiotic treatment for invasive infections in PWID are limited and mainly rely on retrospective studies in populations without a history of injecting drug use.27
An important consideration in the use of oral antimicrobials is the potential for clinically relevant drug interactions. Early oral antimicrobial therapy for invasive infections usually relies on agents with high bioavailability, such as rifampicin, ciprofloxacin and linezolid. These antimicrobials can interact with other medications (including OAT) and recreational drugs.27 Pharmacists should be involved in care teams early to provide dispensing advice and review any potential interactions in conjunction with addiction medicine and pain services. Box 3 provides a summary of some key interactions that may be experienced in the management of PWID with oral antimicrobials. It is also important to consider when the interacting drug is stopped and the need for medications to return to their baseline dose (eg, if rifampicin is used with methadone). At present, there is scant evidence about the comparative effectiveness of early oral antibiotics compared with standard intravenous therapy among PWID with invasive infections. Oral antimicrobials should not be used simply to facilitate an earlier discharge, especially if this would not be standard of care for people without a substance use history. If oral antimicrobials are to be used, a clear management plan with close follow‐up should be implemented to mitigate the risk of non‐completion and loss to follow‐up.
Long‐acting lipoglycopeptides
Lipoglycopeptides, such as dalbavancin and oritavancin, are parenteral antibiotics with long half‐lives that achieve high concentrations in bone, skin and synovium.29,30,31 Dalbavancin can be dosed once weekly thanks to its half‐life of 147–258 hours, while oritavancin can be dosed once per treatment course for gram‐positive infections due to its half‐life of 245–393 hours.30,32 These agents have therefore been suggested as potential management strategies for PWID to provide intravenous antibiotics without the need for prolonged inpatient hospitalisation or OPAT admission.
The United States Food and Drug Administration approved dalbavancin in 2014 and oritavancin in 2015 for the treatment of skin and soft tissue gram‐positive bacterial infections.32,33 There has been increasing interest in the off‐label use of these agents for invasive infections, including in populations who may not be suitable OPAT candidates such as PWID. While current studies are heterogenous regarding the infections treated (including bacteraemia, infective endocarditis, and bone and joint infections), as well as the dosing used, treatment success rates range from 64% to 100%.34,35,36,37,38,39,40,41,42,43,44 Many studies have also demonstrated that these lipoglycopeptides can be successfully used in PWID.36,40,41,44,45 While predominantly retrospective in nature, no significant difference in clinical success and adverse events in PWID subgroups has been found.41,44,45 The spectrum of activity and success in available studies suggest that these agents may have a role to play in the management of invasive infections in PWID. Current limitations of these lipoglycopeptides in the Australian setting include the cost (roughly $1850 per 500 mg vial of dalbavancin) and that they are accessed through the Therapeutic Goods Administration Special Access Scheme and thus need to be imported from overseas, with the associated time lag. However, potential advantages include an alternative to oral therapy and OPAT where there are barriers to these options, earlier discharge from hospital, and decreased health care costs.39 Furthermore, PWID are becoming increasingly familiar with long‐acting preparations thanks to the implementation of long‐acting injectable depot buprenorphine.46 The increasing use of these agents could also allow for the co‐administration of dalbavancin or oritavancin in outpatient settings with weekly depot buprenorphine, again increasing engagement of PWID.
Antimicrobial delivery
Despite increasing evidence for alternative strategies such as OPAT and long‐acting lipoglycopeptides for invasive infections, the default approach for PWID is frequently prolonged inpatient intravenous antimicrobials.13 This should no longer be the default position, as there is increasing evidence that PWID can be discharged safely to an OPAT service with appropriate supports. However, if patients are not able to be managed on OPAT, dalbavancin and oritavancin offer promise as alternatives. The widespread use of these agents is currently limited due to their high cost and lack of availability and predominantly retrospective evidence base. Upcoming randomised controlled trials, such as the DOTS trial (https://clinicaltrials.gov/ct2/show/NCT04775953) assessing dalbavancin efficacy, will strengthen knowledge around their role. Oral antimicrobials may be considered as an alternative option, with close review of all (prescribed and non‐prescribed) pharmaceuticals used to ensure no significant drug interactions will occur. In summary, there are a range of options for the delivery of antimicrobials for PWID with invasive infections, and each case must be considered on its own merit to ensure the best patient‐centred plan is delivered.
Ensuring source control
Antimicrobials are only one component of the successful management of invasive infections. Source control of the infection is required to ensure resolution. However, many barriers remain for PWID to receive surgery. In the case of infective endocarditis, it has traditionally been recommended to avoid surgical management in PWIDs.9,10 However, evidence regarding operative outcomes in IDU‐infective endocarditis is increasing and individual patient‐specific surgical management should be considered.
Similar, or lower, operative mortality and no significant difference in early postoperative mortality has been demonstrated between IDU‐infective endocarditis and infective endocarditis not related to IDU.47,48,49,50 A recent meta‐analysis demonstrated no significant difference in in‐hospital mortality (risk ratio [RR], 0.88; 95% CI, 0.51–1.54) nor in 30‐day mortality (RR, 0.77; 95% CI, 0.36–1.64).47 This contrasts to studies of mid‐ and long term outcomes following surgery, which have demonstrated poorer outcomes in PWID, contributed to by an increased risk of reinfection.48,51,52,53 Survival rates following surgery for IDU‐infective endocarditis in an observational cohort study in Sweden over 17 years demonstrated a 49% 5‐year survival in patients who had IDU‐infective endocarditis compared with 76% in those without a history of injecting, and a higher risk of reoperation (adjusted hazard ratio, 3.47; 95% CI, 1.74–6.89; P < 0.001).48 Overall, these studies demonstrate that PWID can tolerate surgery well and, thus, should be considered for appropriate source control when required. However, the longer term outcomes are complicated due to the increased risk of reinfection. This again indicates the importance of comprehensive care including addressing substance use and addiction during the admission for an IDU‐related invasive infection. Multidisciplinary teams are recommended in guidelines for the management of infective endocarditis9,10 and contribute to improved outcomes in patients.54,55 For PWID, these teams should include not only surgical and medical staff but also addiction medicine, psychiatry, anaesthetics, social work, nursing and peer support as well as the patient themselves so an individualised plan can be made. These teams require collaboration and a willingness to re‐evaluate practices by all specialties and should be spearheaded by change‐makers at institutions to drive their initiation.56 We argue that there should be no defined exclusions to patients receiving surgery, instead the patient and their multidisciplinary team should make an informed decision assessing both medical and psychosocial factors of the individual case at hand.
Management of substance use and psychosocial comorbidities
As well as providing treatment for the invasive infection, an evidence‐based assessment and treatment model should be included for the underlying substance use. Linkage with addiction medicine or alcohol and other drug services is associated with improved outcomes for PWID. This includes increased uptake of OAT, antimicrobial completion, reduced readmission and reduced mortality.57,58,59 Use of OAT by PWID not only reduces illicit opioid use but also overdose risk, injecting related illness including blood‐borne virus transmission, readmission rates, retention in care, and all‐cause mortality.58,60 However, despite documented benefit, hospitalised patients with substance use disorders often have low and delayed referrals to addiction teams and OAT.61,62 Where available, early referral is an essential part of the multidisciplinary care of PWID. Addiction teams are also crucial in supporting patients with stimulant use disorders (such as methamphetamine use), given the variety of use patterns, available preparations, intoxication presentations and potential for prolonged withdrawal period. Referral to addiction medicine should also continue in the post‐discharge period, as there is an increased risk of injecting harms, including overdose, in the period immediately following discharge from hospital.63
Addiction medicine and psychiatry colleagues can also play an important role in educating clinicians regarding addiction and the interplay with past trauma and psychosocial comorbidities on behaviour and engagement. This is particularly important as PWID experience an increased rate of comorbid conditions such as mental health disorders and previous trauma.64 PWID also frequently experience multiple social stressors, including unstable housing, job instability, legal and domestic disputes, which can all negatively affect mental health and substance use risk.64 These comorbid conditions need to be addressed and supported to effectively engage patients and manage their infective complications of IDU. Services including psychiatry, pain specialists, social work and community care, as well as addiction medicine, are thus crucial team members to ensure the adequate acute support of PWID and decrease the longer term risks of injecting‐related harms.64
Early discharge planning
PWID have higher rates of unplanned discharge than non‐IDU patients admitted for the same infective conditions, with a prevalence range of 25–30%.65,66 Unplanned discharge is linked with higher 30‐day mortality, readmission and longer subsequent hospital stays.67,68 Rather than reflecting non‐compliance on the side of the patient, unplanned discharge has more recently been reframed as a failure of the health service to provide a supportive health care environment within which the needs of patients admitted to hospital with IDU, including addiction, mental health and social stressors, are not met.67 An emergency oral antimicrobial plan (including take‐home naloxone) that can be enacted if a patient decides to discharge earlier than advised can help decrease readmission rates and morbidity.69 Efforts should also be made to provide the patient with outpatient follow‐up with infectious diseases specialists or their local doctor, even if they have an unplanned discharge.
Hospital admission as an opportunity for prevention
Screening
There are higher rates of sexually transmissible infections, viral hepatitis, human immunodeficiency virus (HIV) infection, and tuberculosis seen in PWID than non‐PWID.70 Hospital admission can provide the opportunity for PWID, who may not be receiving regular health care, to be screened for these conditions. If positive results are returned, patients should be provided with counselling about the benefits of rapid initiation of therapy, especially for hepatitis C and HIV infection.70 Men who have sex with men and who are negative for HIV infection should also be provided with information about HIV pre‐exposure prophylaxis as well as regular sexually transmissible infection screening.71
Vaccination
Hospitalisation can also be a time to review protection against vaccine‐preventable diseases. In PWID, this includes hepatitis A and B, influenza, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, and tetanus if there is no evidence of immunity or the recommended vaccine guidelines have not been met.70 Pneumococcal vaccines should also be considered if eligible.
Safer injecting practices
Safer injection techniques should be discussed, such as injection site preparation and use of filters and sterile water, where possible, and use of sterile needles and syringes, including not reusing needles.70 Overdose prevention education, such as naloxone information for people who inject opioids, should also be provided, as well as provision of community resources for addiction treatment and the availability of medically supervised injecting rooms. While naloxone is currently available in Australia, there is low access and training with naloxone among Australian PWID.72 Hospital admission thus provides an opportunity to provide take‐home naloxone and associated training, which can thereby also increase accessible naloxone in the community. By promoting harm reduction during admissions, PWID may feel less stigma and judgement, which in turn improves overall engagement in treatment and reduced risk of subsequent admissions with IDU‐related infections.
Conclusion and future research
The management of invasive infections in PWID can be challenging for both patients and clinicians due to the requirement of prolonged antimicrobials and the demands of complex comorbid conditions. Much of the current evidence is driven by retrospective studies, and prospective trials are required to provide strengthened evidence regarding the optimal management of invasive infections in PWID. A prospective multicentre cohort study is currently enrolling in Australia to assess the outcomes of varied management options of PWID admitted to hospital with invasive infections.73 A randomised controlled trial is recruiting in Canada to assess oral antimicrobials for PWID with infective endocarditis (https://clinicaltrials.gov/ct2/show/NCT04544306?draw=2), while an international randomised adaptive clinical trial assessing interventions for Staphylococcus aureus bacteraemia will include a subanalysis of PWID (https://clinicaltrials.gov/ct2/show/NCT05137119).74
The prevalence and diversity of invasive infections in PWID often requires a wide range of health care teams to provide care for these patients. Thus, the use of multidisciplinary teams can not only support patients, but clinicians as well. There is increasing evidence that PWID do not need to remain hospitalised for prolonged periods due to an invasive infection and, therefore, early discussions regarding discharge planning should occur with patients. Applying early use of a multidisciplinary, pragmatic, patient‐centred, non‐judgemental approach may allow these patients to not only achieve improved outcomes for their invasive infections but also reduce their risk of subsequent admissions.
Box 1 – Priorities of care for people who inject drugs (PWID) admitted to hospital with invasive infections
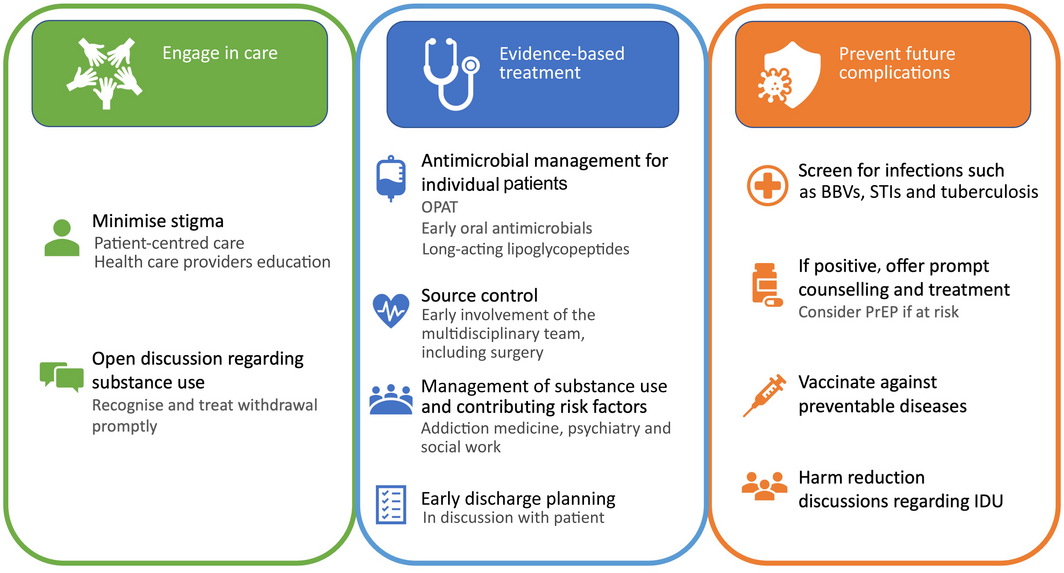
BBV = blood‐borne virus; IDU = injecting drug use; OPAT = outpatient parenteral antimicrobial therapy; PrEP = pre‐exposure prophylaxis; STI = sexually transmissible infection.
Box 2 – Components of a drug history in people who inject drugs admitted with an invasive infection
|
|
|||||||||||||||
Components of a drug history:
|
|||||||||||||||
Common substances to screen for:
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – Interactions between opioid agonist therapy, diazepam and common antimicrobials used in invasive infections
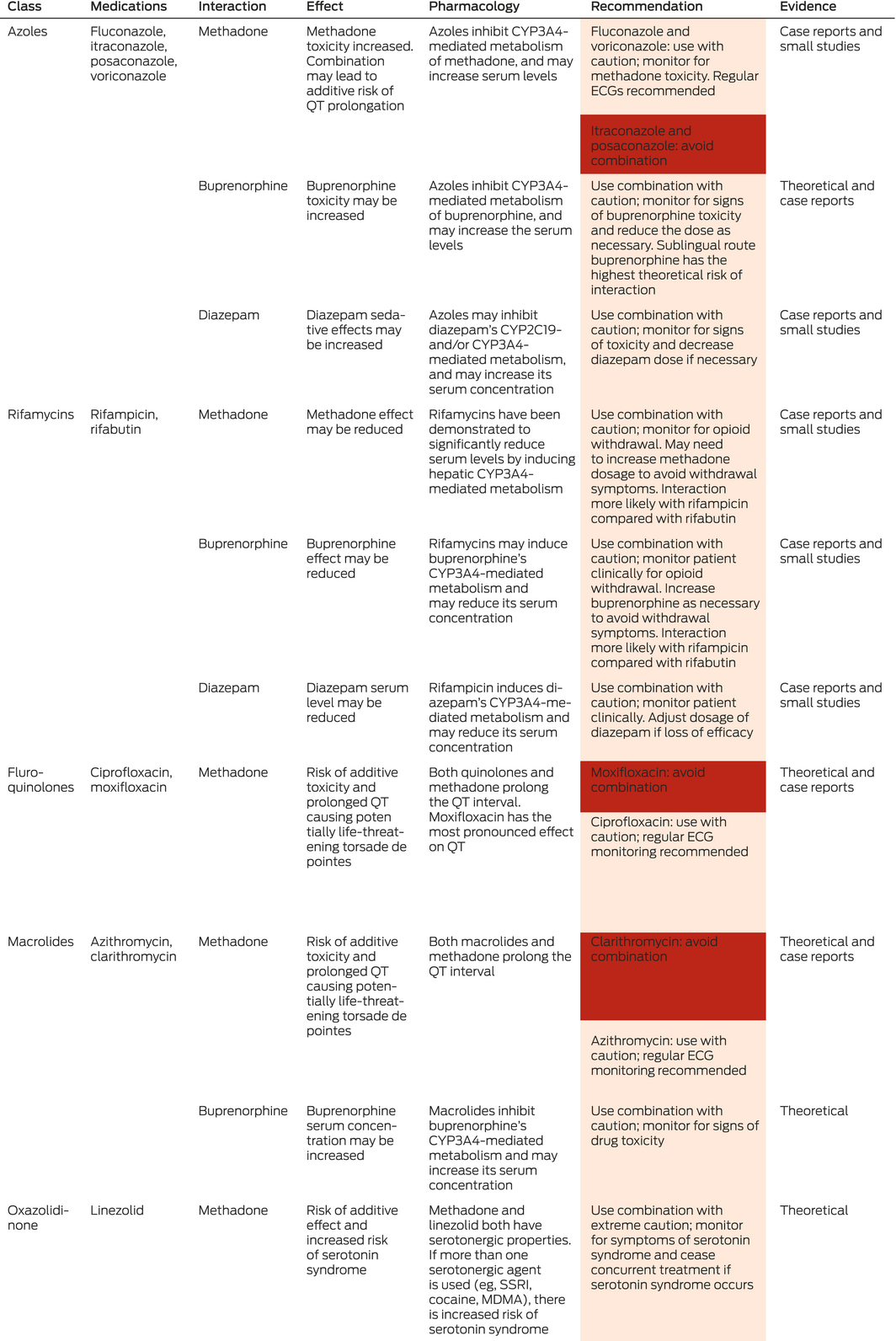
ECG = electrocardiogram, MDMA = 3,4‐Methylenedioxymethamphetamine; SSRI = selective serotonin reuptake inhibitor. Orange colour = use combination with caution; red colour = avoid combination. Data synthesised and adapted from MIMS Online (www.mimsonline.com.au), Australian Medicines Handbook Online (https://amhonline.amh.net.au/auth) and Stockley’s Drug Interactions.28 The references supporting the level of evidence provided are listed in the Supporting Information.
Provenance: Not commissioned; externally peer reviewed.
- Lucy O Attwood1,2
- Megan McKechnie1
- Olga Vujovic1,2
- Peter Higgs3,4
- Martyn Lloyd‐Jones1
- Joseph S Doyle1,2,3
- Andrew J Stewardson1,2
- 1 Alfred Health, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 Burnet Institute, Melbourne, VIC
- 4 La Trobe University, Melbourne, VIC
Open access
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
The authors acknowledge the work of Thuy Bui and Kelly Cairns for their assistance reviewing the pharmacology in this article. The Burnet Institute acknowledges support from the Victorian Government Operational Infrastructure Fund. Lucy Attwood receives postgraduate support from the Australian National Health and Medical Research Council (NHMRC). Joseph Doyle and Andrew Stewardson receive Fellowship support from the NHMRC.
Peter Higgs has received investigator‐driven research funding from Gilead Sciences and AbbVie for work on hepatitis C unrelated to this manuscript. Martyn Lloyd‐Jones has received honoraria for giving lectures and educational sessions organised by Indivior. Joseph Doyle’s institution has received investigator‐initiated research funding from Gilead Sciences and AbbVie and honoraria from Gilead Sciences and AbbVie.
- 1. See I, Gokhale RH, Geller A, et al. National public health burden estimates of endocarditis and skin and soft‐tissue infections related to injection drug use: a review. J Infect Dis 2020; 222 (Suppl 5): S429‐S436.
- 2. Coyle JR, Freeland M, Eckel ST, Hart AL. Trends in morbidity, mortality, and cost of hospitalizations associated with infectious disease sequelae of the opioid epidemic. J Infect Dis 2020; 222 (Suppl 5): S451‐S457.
- 3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013; 131: 23‐35.
- 4. Chan Carusone S, Guta A, Robinson S, et al. “Maybe if I stop the drugs, then maybe they’d care?” — hospital care experiences of people who use drugs. Harm Reduct J 2019; 16: 16.
- 5. Harvey L, Boudreau J, Sliwinski SK, et al. Six Moments of infection prevention in injection drug use: an educational toolkit for clinicians. Open Forum Infect Dis 2022; 9: ofab631.
- 6. Lennox R, Lamarche L, O’Shea T. Peer support workers as a bridge: a qualitative study exploring the role of peer support workers in the care of people who use drugs during and after hospitalization. Harm Reduct J 2021; 18: 19.
- 7. Bassuk EL, Hanson J, Greene RN, et al. Peer‐delivered recovery support services for addictions in the United States: a systematic review. J Subst Abuse Treat 2016; 63: 1‐9.
- 8. Voon P, Callon C, Nguyen P, et al. Denial of prescription analgesia among people who inject drugs in a Canadian setting. Drug Alcohol Rev 2015; 34: 221‐228.
- 9. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075‐3128.
- 10. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435‐1486.
- 11. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56: e1‐e25.
- 12. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61: e26‐e46.
- 13. Rapoport AB, Fischer LS, Santibanez S, et al. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5: ofy132.
- 14. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68: e1‐e35.
- 15. Mitchell ED, Czoski Murray C, Meads D, et al. Clinical and cost‐effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017; 7: e013560.
- 16. Suzuki J, Johnson J, Montgomery M, et al. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5: ofy194.
- 17. Buehrle DJ, Shields RK, Shah N, et al. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis 2017; 4: ofx102.
- 18. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother 2010; 65: 2641‐2644.
- 19. Camsari UM, Libertin CR. Small‐town America’s despair: infected substance users needing outpatient parenteral therapy and risk stratification. Cureus 2017; 9: e1579.
- 20. Vazirian M, Jerry JM, Shrestha NK, Gordon SM. Outcomes of outpatient parenteral antimicrobial therapy in patients with injection drug use. Psychosomatics 2018; 59: 490‐495.
- 21. Dobson PM, Loewenthal MR, Schneider K, Lai K. Comparing injecting drug users with others receiving outpatient parenteral antibiotic therapy. Open Forum Infect Dis 2017; 4: ofx183.
- 22. O’Callaghan K, Tapp S, Hajkowicz K, et al. Outcomes of patients with a history of injecting drug use and receipt of outpatient antimicrobial therapy. Eur J Clin Microbiol Infect Dis 2019; 38: 575‐580.
- 23. D’Couto HT, Robbins GK, Ard KL, et al. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis 2018; 5: ofy056.
- 24. Wald‐Dickler N, Holtom PD, Phillips MC, et al. Oral is the new IV. Challenging decades of blood and bone infection dogma: a systematic review. Am J Med 2022; 135: 369‐379.
- 25. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380: 415‐424
- 26. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380: 425‐436.
- 27. Martinez AE, Scheidegger C, Bättig V, Erb S. Oral antibiotic therapy in people who inject drugs (PWID) with bacteraemia. Swiss Med Wkly 2020; 150: w20259.
- 28. Medicines Complete. Stockley’s drug interactions [website]. London: Royal Pharmaceutical Society, 2022. https://about.medicinescomplete.com/publication/stockleys‐drug‐interactions/ (viewed Apr 2022).
- 29. Cooper CC, Stein GE, Mitra S, et al. Long‐acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt) 2021; 22: 771‐779.
- 30. Zhanel GG, Calic D, Schweizer F, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs 2010; 70: 859‐886.
- 31. Leighton A, Gottlieb AB, Dorr MB, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 2004; 48: 940‐945.
- 32. Rubino CM, Bhavnani SM, Moeck G, et al. Population pharmacokinetic analysis for a single 1200‐milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother 2015; 59: 3365‐3372.
- 33. Boucher HW, Wilcox M, Talbot GH, et al. Once‐weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 2014; 370: 2169‐2179.
- 34. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram‐positive infective endocarditis: 2‐year experience at the General Hospital of Vienna. Clin Infect Dis 2018; 67: 795‐798.
- 35. Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life dalbavancin use in gram‐positive infections. Int J Infect Dis 2019; 81: 210‐214.
- 36. Bryson‐Cahn C, Beieler AM, Chan JD, et al. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019; 6: ofz028.
- 37. Morrisette T, Miller MA, Montague BT, et al. On‐ and off‐label utilization of dalbavancin and oritavancin for gram‐positive infections. J Antimicrob Chemother 2019; 74: 2405‐2416.
- 38. Dinh A, Duran C, Pavese P, et al. French national cohort of first use of dalbavancin: a high proportion of off‐label use. Int J Antimicrob Agents 2019; 54: 668‐672.
- 39. Hidalgo‐Tenorio C, Vinuesa D, Plata A, et al. DALBACEN cohort: dalbavancin as consolidation therapy in patients with endocarditis and/or bloodstream infection produced by gram‐positive cocci. Ann Clin Microbiol Antimicrob 2019; 18: 30.
- 40. Ahiskali A, Rhodes H. Oritavancin for the treatment of complicated gram‐positive infection in persons who inject drugs. BMC Pharmacol Toxicol 2020; 21: 73.
- 41. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram‐positive infections. Infect Dis Ther 2019; 8: 171‐184.
- 42. Tobudic S, Forstner C, Burgmann H, et al. Real‐world experience with dalbavancin therapy in gram‐positive skin and soft tissue infection, bone and joint infection. Infection 2019; 47: 1013‐1020.
- 43. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: A randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019; 6: ofy331.
- 44. Vazquez Deida AA, Shihadeh KC, Preslaski CR, et al. Use of a standardized dalbavancin approach to facilitate earlier hospital discharge for vulnerable patients receiving prolonged inpatient antibiotic therapy. Open Forum Infect Dis 2020; 7: ofaa293.
- 45. Morrisette T, Miller MA, Montague BT, et al. Long‐acting lipoglycopeptides: “lineless antibiotics” for serious infections in persons who use drugs. Open Forum Infect Dis 2019; 6: ofz274.
- 46. Lagios K. Buprenorphine: extended‐release formulations “a game changer”! [letter]. Med J Aust 2021; 214: 534. https://www.mja.com.au/journal/2021/214/11/buprenorphine‐extended‐release‐formulations‐game‐changer#:~:text=To%20the%20Editor%3A%20There%20is
- 47. Hall R, Shaughnessy M, Boll G, et al. Drug use and postoperative mortality following valve surgery for infective endocarditis: A systematic review and meta‐analysis. Clin Infect Dis 2019; 69: 1120‐1129.
- 48. Bearpark L, Sartipy U, Franco‐Cereceda A, Glaser N. Surgery for endocarditis in intravenous drug users. Ann Thorac Surg 2020; 112: 573‐581.
- 49. Kim JB, Ejiofor JI, Yammine M, et al. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg 2016; 152: 832‐841.
- 50. Rudasill SE, Sanaiha Y, Mardock AL, et al. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol 2019; 73: 559‐570.
- 51. Straw S, Baig MW, Gillott R, et al. Long‐term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis 2020; 71: 564‐571.
- 52. Suzuki J, Johnson JA, Montgomery MW, et al. Long‐term outcomes of injection drug‐related infective endocarditis among people who inject drugs. J Addict Med 2020; 14: 282‐286.
- 53. Wurcel AG, Boll G, Burke D, et al. Impact of substance use disorder on midterm mortality after valve surgery for endocarditis. Ann Thorac Surg 2020; 109: 1426‐1432.
- 54. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before‐and‐after study. Open Heart 2017; 4: e000699.
- 55. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis 2019; 6: ofz308.
- 56. Weimer MB, Falker CG, Seval N, et al. The need for multidisciplinary hospital teams for injection drug use‐related infective endocarditis. J Addict Med 2021; doi: https://doi.org/10.1097/ADM.0000000000000916 [Epub ahead of print].
- 57. Wakeman SE, Metlay JP, Chang Y, et al. Inpatient addiction consultation for hospitalized patients increases post‐discharge abstinence and reduces addiction severity. J Gen Intern Med 2017; 32: 909‐916.
- 58. Marks LR, Munigala S, Warren DK, et al. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis 2019; 68: 1935‐1937.
- 59. Santo T, Clark B, Hickman M, et al. Association of opioid agonist treatment with all‐cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta‐analysis. JAMA Psychiatry 2021; 78: 979‐993.
- 60. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ 2017; 357: j1550.
- 61. Rosenthal ES, Karchmer AW, Theisen‐Toupal J, et al. Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis. Am J Med 2016; 129: 481‐485.
- 62. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use‐associated Staphylococcus aureus bacteremia. Open Forum Infect Dis 2019; 6: ofz289.
- 63. Lewer D, Eastwood B, White M, et al. Fatal opioid overdoses during and shortly after hospital admissions in England: A case‐crossover study. PLoS Med 2021; 18: e1003759.
- 64. Colledge S, Larney S, Peacock A, et al. Depression, post‐traumatic stress disorder, suicidality and self‐harm among people who inject drugs: a systematic review and meta‐analysis. Drug Alcohol Depend 2020; 207: 107793.
- 65. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno‐epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med 2014; 105: 59‐66.
- 66. Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health 2015; 105: e53‐e59.
- 67. Ambasta A, Santana M, Ghali WA, Tang K. Discharge against medical advice: “deviant” behaviour or a health system quality gap? BMJ Qual Saf 2020; 29: 348‐352.
- 68. Glasgow JM, Vaughn‐Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30‐day mortality and hospital readmission. J Gen Intern Med 2010; 25: 926‐929.
- 69. Marks LR, Liang SY, Muthulingam D, et al. Evaluation of partial oral antibiotic treatment for persons who inject drugs and are hospitalized with invasive infections. Clin Infect Dis 2020; 71: e650‐e656.
- 70. Centers for Disease Control and Prevention (CDC). Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the US Department of Health and Human Services. MMWR Recomm Rep 2012; 61: 1‐40.
- 71. Australasian Society of HIV, Viral Hepatitis and Sexual Health Medicine. PrEP guidelines update. Prevent HIV by prescribing PrEP. Sydney: ASHM, 2021. https://www.ashm.org.au/resources/hiv‐resources‐list/prep‐guidelines‐2019/ (viewed Apr 2022).
- 72. Conway A, Valerio H, Peacock A, et al. Non‐fatal opioid overdose, naloxone access, and naloxone training among people who recently used opioids or received opioid agonist treatment in Australia: the ETHOS Engage study. Int J Drug Policy 2021; 96: 103421.
- 73. Stewardson AJ, Attwood LO, Doyle JS, et al. Epidemiology and management of invasive infections among people who use drugs (EMU). Australian Society for Infectious Diseases, 2021. https://www.asid.net.au/groups/endorsed‐studies (viewed Apr 2022).
- 74. 74 Staphylococcus aureus Network Adaptive Platform. SNAP trial: governance [website]. SNAP Trial, 2021. https://www.snaptrial.com.au/governance (viewed May 2022).






Summary