Tobacco smoking by pregnant women has negative consequences for both the women and their babies.1 The prevalence of smoking during pregnancy is more than three times as high among pregnant Aboriginal and Torres Strait Islander (Indigenous) women as for other pregnant women in Australia (44% v 12%).2 Although pregnancy is a window of opportunity for altering health‐related behaviour, few health care professionals are trained in providing smoking cessation care for pregnant women,3,4 and they are consequently less likely to assist pregnant women to stop smoking.5 Indigenous women encounter additional barriers if culturally appropriate services are not available.6
Training health care providers in evidence‐based smoking cessation care can improve general quit rates.7 A Cochrane meta‐analysis found low certainty evidence that nicotine replacement therapy (NRT) increases smoking cessation rates during late pregnancy, but without an effect on birth outcomes.8 Many smoking cessation guidelines recommend NRT for pregnant women if non‐pharmacological methods have failed;8,9 Royal Australian College of General Practitioners guidelines prefer oral NRT to patches for pregnant women.10
In this article, we report the protocol of a cluster randomised controlled trial (RCT) of Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT), an intervention in which health care professionals are trained to provide culturally competent smoking cessation care to pregnant Indigenous women, using both behaviour change techniques and pharmacotherapy. Building on our previous research,11,12 the intervention was developed over many years, including an extensive Indigenous community consultation and co‐development phase, testing of printed intervention training resources in three states, and the Indigenous Counselling and Nicotine (ICAN) QUIT in Pregnancy pilot study in six Aboriginal Medical Services in New South Wales, South Australia, and Queensland.13
Study timetable (final) and sites
Changes in the timetable in the course of the study are summarised in the Supporting Information, part 1.
▪ February 2016: Initial ethics approval for the addition of Phase III‐SISTAQUIT RCT to the ICAN QUIT in Pregnancy study by the University of Newcastle (H‐2015‐0438)
▪ February and May 2018: Primary ethics approval for Phase III‐SISTAQUIT RCT study by the Aboriginal Health and Medical Research Council of NSW (1140/15) and the University of Newcastle (H‐2015‐0438).
▪ April 2017 – February 2021: Trial governance established by SISTAQUIT team with each participating health service (local consultation and approvals, trial management training, financial arrangements).
▪ February 2018 – March 2021: Randomisation of practices and training of health practitioners.
▪ August 2018 – December 2021: Recruitment of pregnant women and commencement of data collection.
▪ March 2022: Data collection completed (follow‐up of final participating woman and her baby completed).
▪ July 2022: Data analysis to be completed.
▪ December 2022: Feedback of study findings to participating Aboriginal Medical Services, general practices and hospitals, and to Aboriginal and Torres Strait Islander communities.
Twenty‐one health services (Aboriginal Community Controlled Health Services, Aboriginal Medical Services, general practices, public hospitals) participated in the study. The list of study sites is included in the Supporting Information, part 1.
Methods and analysis
Study design
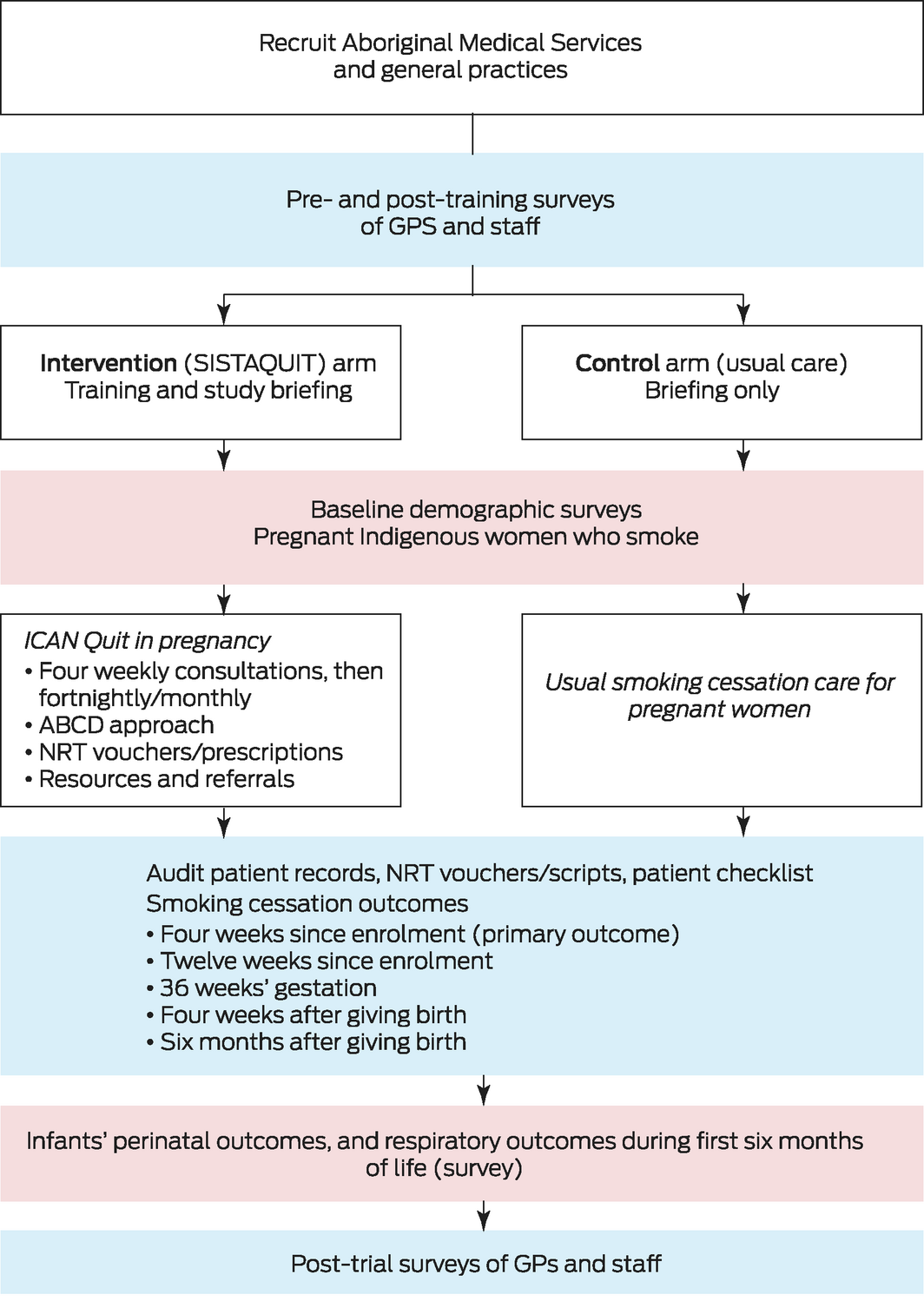
SISTAQUIT is a multicentre, hybrid type 1, pragmatic, cluster RCT that compares the effects of an intervention for improving smoking cessation by pregnant Indigenous women with usual care; it also incorporates efficacy measures and process evaluations.14 The trial, built on the ICAN pilot study,11 is coordinated by University of Newcastle research staff (GG, JF, NR, LS), and the research team includes both Indigenous and non‐Indigenous investigators and research staff. The trial was prospectively registered with the Australia New Zealand Clinical Trials Registry, ACTRN12618000972224 (8 June 2018; amended 6 June 2019).
Aims of the study
The primary outcome is the proportion of pregnant Indigenous women who have quit smoking (self‐reported, and confirmed by carbon monoxide breath testing when possible) four weeks after enrolment. As secondary outcomes, we examined:
▪ smoking cessation rates in pregnant Indigenous women twelve weeks after enrolment, at 36 weeks’ gestation, and four weeks and six months after giving birth;
▪ perinatal outcomes and the incidence of respiratory illness in Indigenous babies during the first six months of life;
▪ health outcomes for women during pregnancy and at the birth of their children;
▪ self‐reported number of quit attempts and cessation rates and the incidence of smoking relapse;
▪ the proportion of health care providers who assisted pregnant Indigenous women with smoking cessation;
▪ NRT provision by the health care provider, including on prescription and vouchers provided by SISTAQUIT and adherence to NRT by pregnant Indigenous women; and
▪ self‐reported use and adherence to NRT for assisting smoking cessation.
Participant recruitment and randomisation
After collating a sampling frame of health services in New South Wales, Queensland, the Northern Territory, Western Australia, and Victoria, including Aboriginal Community Controlled organisations, Aboriginal Medical Services, and general practices, we invited each organisation to participate in SISTAQUIT by email or telephone and through organisational and college newsletters and personal networks. Health care services, stratified according to the Australian Statistical Geographical Classification: Remoteness Area (ASGC‐RA),15 were randomised by independent statisticians, using block randomisation and computer‐generated random number sequences, to the intervention and control groups. Services were randomised in blocks of at least eight sites to balance site characteristics during randomisation. To avoid cross‐contamination, we ensured that services in different trial groups were located at least 30 km apart. Each participating service was reimbursed for the costs of research activities undertaken for the trial.
Health care providers employed at participating services and 0–21 pregnant Indigenous women (32 weeks’ gestation or earlier) at each service were recruited for the study. The women were recruited by an on‐site research facilitator or health care professional trained for this purpose by the research team; women could be recruited at any point during their pre‐natal care. Each participating health care provider and woman provided written informed consent to participation (Box 1, Box 2).
Health services and providers knew their allocation groups, but were asked to not disclose this information to participating women.
Sample size
Our original sample size calculation assumed a standard health care provider training RCT7 and recruitment of about thirty health services. The revised power calculation, based on the recruitment of twenty health services (as recruited nearly two years into the trial) indicated that a sample of 300 women would confer 85% power to detect a 10 percentage point difference in the proportion of abstinent women in the two groups (anticipated control service proportion: 3%16,17) (α = 0.05; intra‐class correlation, 0.0218). Assuming each of the 20 services received at least 20 eligible pregnant Indigenous smokers each year, and that 70% agreed to participate, it was expected that about 280 eligible participants would be available during the first twelve months of the trial.
Study support (control and intervention groups)
All health services received study support from research team members, including:
▪ On‐site study briefing for participating health care providers and managers in trial methods (protocol, data collection methods, informed consent process).
▪ One‐day research facilitator training (intervention arm: = SISTAQUIT health care provider training session; face‐to‐face or videoconference) in good clinical practice, trial methodology (eg, how to obtain informed consent, administer surveys), and conduct of the study.
▪ Ongoing trial support: fortnightly emails, monthly telephone and/or monthly videoconference meetings with the research facilitator.
▪ Post‐study reports, prepared in collaboration with the participating services.
Intervention group services
Intervention group health care providers and services received smoking cessation care training in pre‐recorded webinars delivered by an Indigenous team member, an educational resource package (including PowerPoint slides for the webinars, a treatment manual and flipchart for the health care provider, and a patient booklet for the women), access to free oral NRT for participating pregnant women, implementation support, and a study briefing (trial implementation training). The cessation strategy was based on the ABCD approach to smoking cessation care for Indigenous women (ask/assess; brief advice; cessation; discuss psychosocial context).19
Oral NRT in various formats (eg, oral spray, lozenges) was supplied at the participating services, as NRT uptake is aided by having supplies available on site, and by catering to individual delivery preferences rather than relying on PBS‐subsidised NRT patches, available on prescription. We organised a system in which NRT vouchers were redeemable at a local pharmacy or directly from participating services, facilitating open‐label free supply of oral NRT. When feasible, services were offered the choice of intervention training in person or by videoconference (Zoom), and as one or two sessions. Video recordings of training sessions were available for new and existing staff members, and telephone, email, and videoconference sessions and webinars were offered to staff members who had queries. Health care providers who completed the training course could apply for continued professional development (CPD) points from applicable organisations (Box 3).
Control group services
Control group health care providers and services provided usual smoking cessation care and resources, including NRT subsidised by the PBS or, when applicable, the Close the Gap PBS Co‐payment program.20 The SISTAQUIT intervention was made available to control group services after their participation in the study had ended (ie, after their last participant had given birth).
Primary outcome
The primary outcome is self‐reported smoking cessation by pregnant Indigenous women four weeks after recruitment, confirmed by carbon monoxide testing when possible. During the coronavirus disease 2019 (COVID‐19) pandemic (from March 2020), staff were advised by some services to not breath test participants. Participants were asked about their smoking status, and health care providers assessed the exhaled carbon monoxide of all participating women with a piCO Baby monitor (Bedfont); a value below six parts per million was deemed to confirm abstinence (Box 4).21
Secondary outcomes: health care service‐ and provider‐related outcomes
Process evaluations will assess reach and context, translation of knowledge, and intervention fidelity and feasibility, and compare the impact of SISTAQUIT with that of usual care.
We will assess the proportion of health care practitioners who offered smoking cessation care to pregnant women up to twelve months prior to recruitment to the SISTAQUIT study, the knowledge, attitudes, and self‐reported practices of health care providers in pre‐ and post‐training surveys, the numbers of NRT prescriptions and vouchers provided, and the number of follow‐up visits; we will assess the use of the ABCD approach and behaviour change techniques during smoking cessation consultations, applying a general inductive approach.22
At the middle (at least two years into the study) and at the end of the study, implementation factors will be assessed by Indigenous investigators external to our study team (Box 4).
Secondary outcomes: mother‐ and infant‐related outcomes
We also assessed smoking cessation twelve weeks after recruitment to the study, at 36 weeks’ gestation, four weeks after giving birth, and six months after giving birth; the number of quit attempts and self‐reported quit rates; relapse to smoking; adherence to NRT treatment advice; and health outcomes for participating mothers during pregnancy and at birth (eg, from hospital reports and discharge summaries).
We will assess perinatal outcomes and episodes of respiratory illness in babies born to participating women who consent to these assessments during the first six months of life. Information on perinatal outcomes, including length, weight, and clinical assessment at birth and at six months, will be obtained from health care service records. Respiratory illness‐related items, including cough and wheeze, ear infections, doctors’ visits, hospital admissions, medications, and exposure to cigarette smoke, will be assessed each month in a 14‐item survey. The survey also includes questions about the cost and affordability of medications and out‐of‐pocket medical expenses.23
Data analysis
Quantitative data will be analysed in R 4.1 (R Foundation for Statistical Computing), qualitative data in NVivo 12 (QSR International). The baseline characteristics of participating women and health care providers will be reported as means with standard deviations (continuous data) or counts and proportions (categorical data).
Quit rates (primary and secondary outcomes)
In intention‐to‐treat analyses, we will compare quit rates for the intervention and control groups at four weeks (primary outcome), and 7‐day abstinence at follow‐up time points (secondary outcomes) in mixed effects logistic regression models. The model will include time and treatment group as fixed categorical effects, an interaction term (treatment group × time), stratification variable (geographic location of health service), and random intercepts for health service and participant (clustered by site, with repeated measures over time). Multiple imputation (logical inference of missing smoking status, using the chained regression equations method) will be the primary method of dealing with missing data, under the assumption that they are missing at random. The adjusted relative difference between treatment groups (expressed as an odds ratio with 95% confidence interval) will be reported for each time point. Sensitivity analyses will assess the robustness of estimated treatment effects for the primary outcome by adjusting for important patient or provider characteristics that appear unbalanced between groups, and applying different methods for handling missing data (eg, pattern mixture models).
Secondary outcomes: health services
The numbers of women who attend each follow‐up session and of health care providers who attend each webinar session will be reported as the respective proportions of eligible participants. Reach will be reported as the proportions of eligible health care providers and women who participate in the trial, and the national and regional representativeness of the participating health care providers.
Intervention fidelity will be assessed on the basis of responses to the health practitioner surveys, the number of webinars delivered as intended (as recorded by research team and research facilitators), and the use of behaviour change techniques.
Implementation challenges will be assessed mid‐study (at least two years into the study) and at the end of the study in telephone/video interviews or in surveys by external researchers. The qualitative data will be assessed in a general inductive analysis and a framework analysis using the Behaviour Change Wheel.12
Secondary outcomes: health care providers
Changes from baseline to follow‐up in the knowledge, attitudes, and practices of health care providers in each group regarding smoking cessation by pregnant women will be compared in linear mixed effects regression models with the same fixed and random effects as the primary outcome model.
We will assess the number and type of behaviour change techniques used by health care providers, using professionally transcribed recorded interviews. Two behaviour change technique coders will independently code up to 72 consultations with participating women, applying the taxonomy of smoking cessation behaviour change techniques24 and the coding framework developed for our pilot study.25
The numbers of care elements delivered as intended (consultations, NRT vouchers issued, resources provided to women) and resources used by health care providers will be summarised by treatment group as counts and proportions or means with standard deviations.
Secondary outcomes: women
Smoking relapse and the use of and adherence to NRT in the two groups will be assessed in a mixed effects logistic regression model with the same fixed and random effects as the primary outcome model.
Perinatal outcomes and respiratory outcomes, based on survey responses for the two groups, will be reported as means with standard deviations or medians with interquartile ranges (continuous data) or frequencies and proportions (categorical data). Further statistical analysis will not be undertaken because of the low anticipated response rate.
We will also conduct a research impact assessment to increase transparency regarding the successes and limitations of the SISTAQUIT study. This assessment will use the HMRI FAIT framework,26 comprising a Program Logic model, supporting metrics and indicators, and a narrative documentation of the investigation.
Data storage
Baseline and follow‐up participant and health care provider data will be collected in REDCap, a secure web‐based database system. Participating health services will be provided with tablet computers pre‐loaded with REDCap software for collecting participant data. The research facilitators at each health service will store data‐collecting devices and paper data records in locked cabinets accessible only to authorised staff. The REDCap application is hosted locally on physically and virtually secured Hunter Medical Research Institute servers (https://redcap.hmri.org.au).
Ethics approval
The human research ethics committees of the University of Newcastle (H‐2015‐0438) and the Aboriginal Health and Medical Research Council of NSW (1140/15) provided primary ethics approval for the SISTAQUIT trial. It was also approved by those of the Cairns and Hinterland Health Service District (Far North Queensland Human Research Ethics Committee; HREC/18/QCH/27), the Darling Downs Hospital and Health Service (HREC/18/QTDD/10), the Northern Territory Department of Health and Menzies School of Health Research (2017‐2997), the Aboriginal Health Council of WA (Western Australian Aboriginal Health Ethics Committee; 826), and the Aboriginal Health Research Ethics Committee of South Australia (04‐16‐652).
Cultural safety and data monitoring
An Aboriginal advisory panel including members of Aboriginal Community Controlled Health Services, Aboriginal Medical Services, and Indigenous experts is monitoring the conduct of the study and providing cultural advice, and will also monitor the dissemination of its findings. An independent data safety and monitoring board will oversee the integrity and safety of data collection, storage, and sharing.
Author contributions
Gillian Gould was the principal Chief Investigator, supervised the design and implementation of the research, and wrote the original protocol for this project. Nicole Ryan implemented the project design, participated in data collection, oversaw the research team and study, and co‐wrote and critically reviewed the final manuscript. Leah Stevenson and Ratika Kumar implemented the study design, and drafted, reviewed, and approved the final manuscript. Kristin Carson‐Chahhoud and Katherine Boydell were Chief Investigators and supervised study design. Christopher Oldmeadow was the study statistician and supervised study design. Simon Deeming, Christopher Doran and Joerg Mattes drafted and critically reviewed the protocol and supervised study design. Andrew Searles, Louise Atkins, and Marilyn Clarke supervised study design. Joley Foster implemented the study design and coordinated the trial with the health services and research facilitators.
Dissemination of findings
Study findings will be reported to Aboriginal Community Controlled Health Services, Aboriginal Medical Services, general practices, hospitals, and communities participating in the SISTAQUIT study. Newsletters and reports for non‐medical readers, tailored to local needs, will be provided to participating communities. Research findings and their implications will also be disseminated using art‐based strategies (eg, infographics) for Aboriginal research partners and participating Aboriginal Medical Services. Our findings will be reported at state, national, and international conferences, in peer‐reviewed journals, and in public media. The research team will use their extensive professional networks and their organisations’ social media to promote the study locally and overseas. Policy briefs will be formulated and communicated to relevant peak Aboriginal and government organisations, and the major funders (National Health and Medical Research Council and the Global Alliance for Chronic Disease). Data will be published only in aggregate form; that is, individual participating women, health providers, and health services will not be identifiable. De‐identified data will be available on application to the trial coordinators for a period of seven years after completion of the SISTAQUIT trial.
Funding statement
The study is funded by the National Health and Medical Research Council (NHMRC) and the Global Alliance for Chronic Disease (APP11160840), the Cancer Institute of NSW, Cancer Australia, the Cure Cancer Australia Foundation, the University of Newcastle (equipment grant), the University of Newcastle Centre for Brain and Mental Health Research (infrastructure grant), and the Hunter Medical Research Institute. Gillian Gould was supported by a Cancer Institute NSW Early Career Fellowship (2019/COF001), and an NHMRC Translating Research Into Practice Fellowship (15ECF/I‐52).
Acknowledgements
We thank the Aboriginal communities who have contributed to the implementation of the SISTAQUIT RCT study, including the research facilitators, staff, and participating women at the participating Aboriginal Medical Services, general practices, and hospital health services. We also thank the members of the Stakeholder and Consumer Aboriginal Advisory Panel for the SISTAQUIT trial for their ongoing guidance and support in implementing the study.
Received 2 September 2021, accepted 17 February 2022
Box 1 – The Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT) randomised controlled trial: selection and randomisation of participants
ABCD = Ask and Assess, Brief advice, Cessation, Discuss strategy; AMS = Aboriginal Medical Service; GP = general practitioner; NRT = nicotine replacement therapy.
Box 2 – The Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT) randomised controlled trial: inclusion criteria
|
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
* A maximum of two clinics within a service could be included in the trial. † Alternatively, a GP in an associated service or another health provider who could prescribe nicotine replacement therapy could be considered. ‡ Participating women were not obliged to attempt to quit smoking. § Women could also consent to their babies being followed up until six months of age. |
|||||||||||||||
Box 3 – The Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT) randomised controlled trial: health care provider training (intervention arm)
|
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 4 – The Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT) randomised controlled trial: assessments for primary and secondary outcomes
|
|
During pregnancy |
After birth of baby |
|
||||||||||||
|
Assessment (assessor) |
Day 0 |
4 weeks(±3 days) |
2 months |
12 weeks(±7 days) |
36 weeks’ gestation |
4 weeks |
6 months |
12 months |
Middle of study |
End of study |
|||||
|
|
|||||||||||||||
|
Women |
|
|
|
|
|
|
|
|
|
|
|||||
|
Review eligibility (HCP) |
X |
|
|
|
|
|
|
|
|
|
|||||
|
Informed consent (RF) |
X |
|
|
|
|
|
|
|
|
|
|||||
|
Patient survey (RF) |
X |
X |
|
X |
X |
X |
|
|
|
|
|||||
|
Measure carbon monoxide (RF) |
X |
X |
|
X |
X |
X |
|
|
|
|
|||||
|
Clinical file audit |
|
|
|
|
|
|
|
|
|
X |
|||||
|
Audio‐recordings of consultations (optional) (HCP*) |
X |
X |
|
X |
X |
X |
X |
|
|
|
|||||
|
Post‐study interview (external researcher) |
|
|
|
|
|
|
|
|
|
X |
|||||
|
Health care providers |
|
|
|
|
|
|
|
|
|
|
|||||
|
Baseline survey† |
X |
|
|
|
|
|
|
|
|
|
|||||
|
Follow‐up survey† |
|
|
X |
|
|
|
|
|
|
|
|||||
|
Follow‐up survey† |
|
|
|
|
|
|
|
X |
|
|
|||||
|
Interview or survey (external researcher)†,‡ |
|
|
|
|
|
|
|
|
X |
|
|||||
|
Post‐study interview (external researcher) |
|
|
|
|
|
|
|
|
|
X |
|||||
|
Health service staff, research facilitators |
|
|
|
|
|
|
|
|
|
|
|||||
|
Interview or survey (external researcher)†,‡ |
|
|
|
|
|
|
|
|
X |
|
|||||
|
|
|||||||||||||||
|
HCP = health care provider; RF = research facilitator * With assistance from RF. † HCP preference: online or paper. ‡ To ascertain implementation challenges. |
|||||||||||||||
- Gillian S Gould1
- Nicole M Ryan1,2
- Ratika Kumar1
- Leah C Stevenson1
- Kristin V Carson‐Chahhoud3,4
- Christopher Oldmeadow5
- Joley Foster2
- Simon Deeming2
- Katherine Boydell6
- Christopher M Doran7
- Andrew Searles2,5
- Joerg Mattes2
- Louise Atkins8
- Marilyn Clarke2
- 1 Southern Cross University, Coffs Harbour, NSW
- 2 The University of Newcastle, Newcastle, NSW
- 3 The University of Adelaide, Adelaide, SA
- 4 The University of South Australia, Adelaide, SA
- 5 Hunter Medical Research Institute, Newcastle, NSW
- 6 Black Dog Institute, Sydney, NSW
- 7 Central Queensland University, Brisbane, QLD
- 8 UCL Psychology and Language Sciences University College London, London, United Kingdom
Open access
Open access publishing facilitated by Southern Cross University, as part of the Wiley ‐ Southern Cross University agreement via the Council of Australian University Librarians.
No relevant disclosures.
- 1. Gould GS, Havard A, Lim LL, the Psanz Smoking In Pregnancy Expert Group, Kumar R. Exposure to tobacco, environmental tobacco smoke and nicotine in pregnancy: a pragmatic overview of reviews of maternal and child outcomes, effectiveness of interventions and barriers and facilitators to quitting. Int J Environ Res Public Health 2020; 17: 2034.
- 2. Australian Institute of Health and Welfare. Alcohol, tobacco and other drugs in Australia: Aboriginal and Torres Strait Islander people. Updated 20 Apr 2022. https://www.aihw.gov.au/reports/alcohol/alcohol‐tobacco‐other‐drugs‐australia/contents/priority‐populations/aboriginal‐and‐torres‐strait‐islander‐people (viewed Mar 2022).
- 3. Zeev YB, Bonevski B, Twyman L, et al. Opportunities missed: a cross‐sectional survey of the provision of smoking cessation care to pregnant women by Australian general practitioners and obstetricians. Nicotine Tob Res 2017; 19: 636‐641.
- 4. Bar‐Zeev Y, Skelton E, Bonevski B, et al. Overcoming challenges to treating tobacco use during pregnancy: a qualitative study of Australian general practitioners barriers. BMC Pregnancy Childbirth 2019; 19: 61.
- 5. Gould GS, Twyman L, Stevenson L, et al. What components of smoking cessation care during pregnancy are implemented by health providers? A systematic review and meta‐analysis. BMJ Open 2019; 9: e026037.
- 6. Bovill M, Gruppetta M, Cadet‐James Y, et al. Wula (voices) of Aboriginal women on barriers to accepting smoking cessation support during pregnancy: findings from a qualitative study. Women Birth 2018; 31: 10‐16.
- 7. Carson KV, Verbiest MEA, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev 2012; CD000214.
- 8. Claire R, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2020; CD010078.
- 9. Grangé G, Berlin I, Bretelle F, et al. Smoking and smoking cessation in pregnancy. Synthesis of a systematic review. J Gynecol Obstet Hum Reprod 2020; 49: 101847.
- 10. Royal Australian College of General Practitioners. “Supporting smoking cessation: a guide for health professionals”. Updated 29 Sept 2021. https://www.racgp.org.au/clinical‐resources/clinical‐guidelines/key‐racgp‐guidelines/view‐all‐racgp‐guidelines/supporting‐smoking‐cessation (viewed Mar 2022).
- 11. Bar‐Zeev Y, Bovill M, Bonevski B, et al. Indigenous counselling and nicotine (ICAN) Quit in pregnancy: developing an evidence‐based intervention for smoking cessation for indigenous pregnant women [poster abstract]. Asia Pac J Clin Onc 2015; 11 (Suppl 5): 8‐9.
- 12. Gould GS, Bar‐Zeev Y, Bovill M, et al. Designing an implementation intervention with the Behaviour Change Wheel for health provider smoking cessation care for Australian Indigenous pregnant women. Implement Sci 2017; 12: 114.
- 13. Bar‐Zeev Y, Bonevski B, Bovill M, et al; ICAN QUIT in Pregnancy Pilot Group. The Indigenous Counselling and Nicotine (ICAN) QUIT in Pregnancy Pilot Study protocol: a feasibility step‐wedge cluster randomised trial to improve health providers’ management of smoking during pregnancy. BMJ Open 2017; 7: e016095.
- 14. Curran GM, Bauer M, Mittman B, et al. Effectiveness–implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012; 50: 217‐226.
- 15. Australian Department of Health. Australian Statistical Geography Standard: Remoteness Area. Updated 14 Dec 2021. https://www.health.gov.au/health‐topics/rural‐health‐workforce/classifications/asgs‐ra (viewed Mar 2022).
- 16. Eades SJ, Sanson‐Fisher RW, Panaretto K. An intensive smoking intervention for pregnant Aboriginal and Torres Strait Islander women: a randomised controlled trial. Med J Aust 2013; 198: 23‐24. https://www.mja.com.au/journal/2012/197/1/intensive‐smoking‐intervention‐pregnant‐aboriginal‐and‐torres‐strait‐islander
- 17. Passmore E, McGuire R, Correll P, Bentley J. Demographic factors associated with smoking cessation during pregnancy in New South Wales, Australia, 2000–2011. BMC Public Health 2015; 15: 398.
- 18. Adams G, Gulliford MC, Ukoumunne OC, et al. Patterns of intra‐cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004; 57: 785‐794.
- 19. Gould GS, Bittoun R, Clarke MJ. A pragmatic guide for smoking cessation counselling and the initiation of nicotine replacement therapy for pregnant Aboriginal and Torres Strait Islander smokers. J Smok Cessat 2015; 10: 96‐105.
- 20. Australian Department of Health. The Closing the Gap (CTG) PBS Co‐payment program. Updated 17 Feb 2022. https://www.pbs.gov.au/info/publication/factsheets/closing‐the‐gap‐pbs‐co‐payment‐measure (viewed Apr 2022).
- 21. Gomez C, Berlin I, Marquis P, Delcroix M. Expired air carbon monoxide concentration in mothers and their spouses above 5 ppm is associated with decreased fetal growth. Prev Med 2005; 40: 10‐15.
- 22. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2016; 27: 237‐246.
- 23. Perkes S, Bonevski B, Mattes J, et al. Respiratory, birth and health economic measures for use with Indigenous Australian infants in a research trial: a modified Delphi with an Indigenous panel. BMC Pediatr 2020; 20: 368.
- 24. Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011; 36: 315‐319.
- 25. Bar‐Zeev Y, Skeleton E, Bovill M, et al. Feasibility of audio‐recording consultations with pregnant Australian Indigenous women to assess use of smoking cessation behaviour change techniques. J Smok Cessat 2021: 6668748.
- 26. Searles A, Doran C, Attia J, et al. An approach to measuring and encouraging research translation and research impact. Health Res Policy Syst 2016; 14: 1‐3.






Summary
Background: About 44% of Indigenous Australian women smoke during pregnancy, compared with 12% of pregnant non-Indigenous women. Health care providers can assist smoking cessation, but they are not typically trained in culturally appropriate methods.
Objectives: To determine whether a health care worker training intervention increases smoking cessation rates among Indigenous pregnant smokers compared with usual care.
Methods and analysis: Supporting Indigenous Smokers to Assist Quitting (SISTAQUIT) study is a multicentre, hybrid type 1, pragmatic, cluster randomised controlled trial that compares the effects of an intervention for improving smoking cessation by pregnant Indigenous women (16 years or older, 32 weeks’ gestation or less) with usual care. Twenty-one health services caring for Indigenous people in five Australian jurisdictions were randomised to the intervention (ten sites) or control groups (eleven sites). Health care providers at intervention sites received smoking cessation care training based on the ABCD (ask/assess; brief advice; cessation; discuss psychosocial context) approach to smoking cessation for Indigenous women, an educational resource package, free oral nicotine replacement therapy for participating women, implementation support, and trial implementation training. Health care providers in control group services provided usual care. Primary outcome: abstinence from smoking (self-reported abstinence via survey, validated by carbon monoxide breath testing when possible) four weeks after enrolment in the study. Secondary outcomes: health service process evaluations; knowledge, attitudes, and practices of health care providers; and longer term abstinence, perinatal outcomes, and respiratory outcomes for babies (to six months).
Ethics approval: The human research ethics committees of the University of Newcastle (H-2015-0438) and the Aboriginal Health and Medical Research Council of NSW (1140/15) provided the primary ethics approval.
Dissemination of results: Findings will be disseminated in peerreviewed publications, at local and overseas conferences, and via public and social media, and to participating health services in artbased formats and reports. Policy briefs will be communicated to relevant government organisations.
Trial registration: Australia New Zealand Clinical Trials Registry, ACTRN12618000972224 (prospective).