The known: Hereditary pancreatitis typically commences during childhood and leads to lifelong disability and increased risk of pancreatic cancer. Its clinical and genetic features have not been characterised in a population‐based study in Australia.
The new: Twenty‐five of 44 South Australians with confirmed hereditary pancreatitis during 2006–21 were Indigenous people (57%), and the estimated prevalence was much higher for Indigenous Australians than other people (71 v 1.1 per 100 000 population). A large proportion of people with hereditary pancreatitis (86%) required prescribed opioids for pain relief.
The implications: Hereditary pancreatitis should be considered as a cause of early onset pancreatitis, particularly in young Indigenous people.
Hereditary pancreatitis was recognised as a clinical syndrome in 1952,7 and mutations in the cationic trypsinogen (= serine protease 1) gene (PRSS1) on chromosome 7q were identified as major aetiological factors in 1996.7,8,9,10 More than twenty pathogenic PRSS1 mutations have since been described, accounting for 80% of all cases of hereditary pancreatitis in people of European ancestry.7,8,9,10 Other implicated genes include those that encode serine protease inhibitor Kazal‐type 1 (SPINK1, on chromosome 5q32), cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC), and A‐type carboxypeptidase (CPA1).9,10,11 Modifier genes, such as the calcium‐sensing receptor gene (CASR), also influence the risk of hereditary pancreatitis.12
Most recurrent and chronic pancreatitis in children is genetically determined.13 One study found that children with PRSS1 variants are at risk of more rapid progression, exocrine pancreatic insufficiency, and diabetes than those with other pancreatitis‐associated gene mutations.14
In hereditary pancreatitis, inflammation is initially restricted to the acinar and ductal cells, leading to exocrine insufficiency; the pancreatic islets are affected only late in the disease process,1,15 and patients require pancreatic enzyme replacement long before diabetes develops.15,16 As many as 60% of people with symptomatic pancreatitis will ultimately require surgical interventions (including endoscopic procedures) because of severe abdominal pain, with unavoidable loss of pancreatic function.1,2,6,16
In this article, we report the first systematic, population‐based study of hereditary pancreatitis in Australia. We characterised the clinical phenotypes and genetic variants of people diagnosed in South Australia during 2006–21, and used whole exome sequencing to estimate allele frequencies.
Methods
All people with confirmed molecular diagnoses of hereditary pancreatitis from the SA Clinical Genetics Service, SA Pathology, the Women’s and Children’s Hospital, or the Royal Adelaide Hospital during 1 January 2006 – 30 June 2021 were included in our study. We collected saliva from each participant for DNA extraction and genotyping by whole exome sequencing. Informed consent for participation was provided by each person or (for minors) their caregivers or legal guardians.
We used a questionnaire to collect information on personal and family history of hereditary pancreatitis, age at symptom onset, attack frequency, pancreatitis‐related pain, medication use and medical interventions, and alcohol use and smoking history (Supporting Information). Quality of life (physical and mental health) was assessed during 1 April 2019 – 30 June 2021 with the 12‐item Short Form Health Survey (SF‐12).17 Responses for each SF‐12 physical and mental health category were weighted according to the SF‐12 standard scoring manual, and the overall score categorised as no (> 60), light (50–59), moderate (40–49), or high (< 40) impact of pancreatitis on physical or mental health. We also collected information on blood test results, previous genotyping, and medical imaging findings.
DNA was extracted from saliva collected in Oragene OG‐600 kits (DNA Genotek) by the SA Pathology Australian Cancer Research Foundation (ACRF) Cancer Genomics Facility. Library preparation and whole exome sequencing were also undertaken by the ACRF Cancer Genomics Facility. Sequencing data were filtered for variants of any population frequency recognised as pathogenic for hereditary pancreatitis, and for heterozygous rare variants in genes associated with hereditary pancreatitis (allele frequency < 0.01% in the Genome Aggregation Database [gnomAD], https://gnomad.broadinstitute.org) predicted to be pathogenic.
Using linked SA‐NT Datalink administrative data,18 we compared the demographic characteristics of our hereditary pancreatitis group with those of 2504 people with adult‐onset (> 19 years) chronic pancreatitis admitted to South Australian public hospitals during 1 January 2001 – 31 December 2019 (International Statistical Classification of Diseases, tenth revision codes K86.0 [alcohol‐induced chronic pancreatitis] or K86.1 [other chronic pancreatitis]). This included all adults admitted to SA public hospitals with chronic pancreatitis during this period.
All data, including questionnaire responses and medical histories, were entered into a REDCap database. Categorical variables are summarised as frequencies and proportions. Statistical analyses were undertaken in R 4.0.2 (R Foundation for Statistical Computing) and Minitab 20.4.0.
Ethics approval
The human research ethics committees of the Central Adelaide Local Health Network (HREC/18/CALHN/83), the Women’s and Children’s Hospital (HREC/19/WCHN/87), and the SA Department of Health and Wellbeing (HREC/19/SAH/84) approved the study.
Results
We identified 44 people with molecular diagnoses of hereditary pancreatitis (ie, with at least one hereditary pancreatitis‐associated genetic variant) in ten distinct families. All had lived in South Australia at the time of diagnosis, and all were alive at the time of our study. Twenty‐five were Indigenous Australians (57%). In total, 36 people had mutations in PRSS1, five in SPINK1, and three in both PRSS1 and SPINK1 (Box 1, Box 2). As the population of South Australia in December 2020 was 1.771 million,22 the estimated prevalence of genotype‐confirmed hereditary pancreatitis was 2.5 (95% confidence interval [CI], 2.2–2.8) cases per 100 000 population. Based on self‐reported ethnic background, the estimated prevalence was 1.1 (95% CI, 0.72–1.4) cases per 100 000 population for non‐Indigenous South Australians and 71 (95% CI, 66–77) per 100 000 population for Indigenous South Australians.22,23
Six of 44 people with hereditary pancreatitis were younger than 10 years old at the time of study participation, 18 were 10–20 years old, seven were 21–40 years old, and 13 were over 40 years of age. Seventeen participants were male (39%); the median age for male participants (28 years; interquartile range [IQR], 12–33 years) was lower than for the 27 female participants (39 years; IQR, 15–53 years). Onset of symptoms before the age of ten years was reported by 37 participants (84%), and before the age of 20 years by all participants.
In the comparator group of adults admitted to hospital with chronic pancreatitis, 1564 were men (62%) and 299 were Indigenous people (12%). All had been diagnosed after the age of 20 years (median, 53 years; IQR, 41–66 years).
Six people with hereditary pancreatitis (14%) were past or current smokers. Fifteen reported light or moderate alcohol consumption during the twelve months preceding the study (34%), 29 reported abstinence (66%). Fifteen people had diabetes mellitus (34%); eight had undergone surgery related to the progression of pancreatitis (18%); four had histories of cholelithiasis (9%), and seven pancreatic pseudocysts (16%) (Box 3).
Thirty‐eight people (86%) regularly used opioids for pain relief, and 39 described the level of onset pain as moderate or high (89%). The physical impact of pancreatitis‐related pain during the preceding four weeks was described as moderate or high by 35 people (79%) and the mental health impact as moderate or high by 18 people (41%). Thirty‐two people (73%) reported five or more pancreatitis attacks during the preceding twelve months, and twelve (27%) more than ten attacks (Box 4).
None of the people in our study had developed pancreatic cancer. Three participants (from two families) each reported they had one first degree relative with pancreatic cancer who had since died; in each case, the cancer developed while the relative was aged 60–69 years.
Discussion
We have described the first population‐based clinical and genetic characterisation of people with hereditary pancreatitis in Australia. For all 44 people diagnosed in South Australia during 2006–21, symptoms commenced before the age of 20 years, and before the age of ten years in 37 cases (84%). This finding is consistent with reports from the United States8 and Europe19,20,21 that people with hereditary pancreatitis typically first experience symptoms during childhood or early adolescence.
The largest reported hereditary pancreatitis case series, the European Registry of Hereditary Pancreatitis and Pancreatic Cancer, ascertained 418 affected people (112 families, 14 countries) diagnosed during 1997–2004.19 In a study based on a sample of 200 patients in 78 families, its prevalence in France was estimated to be at least 0.3 cases per 100 000 population,20 and the prevalence of symptomatic cases in Denmark was estimated to be 0.57 cases per 100 000 population (38 people in 13 families).21
Our estimated prevalence of hereditary pancreatitis in South Australia (2.5 cases per 100 000 population) is much higher than the rates reported by European studies. Further, the estimated prevalence among Indigenous people (71 per 100 000) was almost 70 times that for non‐Indigenous South Australians (1.1 per 100 000). A recent systematic review and meta‐analysis similarly found that rates of pancreatitis in indigenous populations were higher than in local people of European origin, including a 4.8‐fold higher rate for New Zealand Māori than for New Zealanders of European background.24
As in other reports, the gene most frequently implicated as the sole mutation in our study was PRSS1 (36 of 44 cases, 82%). The most frequent PRSS1 mutations were the R122H (21, 48%) and the N291 variants (14, 32%), comparable with the findings of the large French20 and European case series.19 The PRSS1 R122H and N291 mutations were the only pathogenic variants in Indigenous participants. The SPINK1 N34S variant was implicated in five cases (11%). Loss‐of‐function SPINK1 mutations such as N34S lead to reduced levels of pancreatic secretory trypsin inhibitor, allowing trypsin to initiate pancreatitis.3 The absence of CFTR gene variants in our sample was unsurprising; people of European background (19 of 44 participants) are rarely homozygous for pathogenic variants of this gene. We are currently investigating whether other potentially disease‐causing or predisposing genetic variants can be identified in our sample of people with hereditary pancreatitis.
The small proportion of people who had undergone medical interventions suggests that standard care for hereditary pancreatitis in Australia principally comprises pain management and routine monitoring. Tobacco and alcohol use was low in our sample, consistent with healthy lifestyles that would make most patients suitable for proactive interventions.
The large proportion of Indigenous patients in our study (57%; proportion in the South Australian population, 2016: 2%23) suggests that hereditary pancreatitis is more frequent among Indigenous people than other Australians. Further, 12% of adults admitted to hospital because of chronic pancreatitis were Indigenous people, some of whom may have had undiagnosed hereditary pancreatitis. A 2004 case report25 described an Indigenous family with several members who had hereditary pancreatitis; in our reports on total pancreatectomy and islet auto‐transplantation (TP‐IAT), most patients with hereditary pancreatitis were also Indigenous people.5,16 The prevalence of exocrine pancreas disorders is generally higher in indigenous than European origin populations.24 The need to raise clinical awareness that hereditary pancreatitis should be considered when diagnosing pancreatitis in younger Indigenous Australians is an important pragmatic implication of our study. The lack of awareness of hereditary pancreatitis amongst South Australian medical practitioners was noted by several participants.
The high prevalence of hereditary pancreatitis, and particularly of PRSS1 mutations, among Indigenous Australians may have one or more explanations: for example, founder effects, leading to reduced genetic variation; a population bottleneck caused by an abrupt decline in population size, randomly increasing the relative frequency of some gene variants; or a different PRSS1 pseudogene architecture in Indigenous people that facilitates more frequent gene conversion events.
Fifteen people in our study had diabetes mellitus (34%), and similar rates of type 3c diabetes among people with hereditary pancreatitis have been reported in Europe (France, 36%;20 Denmark, 32%21). Diabetes secondary to chronic pancreatic inflammation might be averted by TP‐IAT, a new treatment in which the pancreas is removed, and its islet cells are isolated and infused into the liver. Our experience with this procedure is that, if undertaken early, it can greatly improve the quality of life of people with hereditary pancreatitis in terms of pain relief and prevention of type 3c diabetes.5,6,16 Twenty‐nine people in our study (66%) fulfilled the Minnesota criteria for the appropriateness of TP‐IAT for patients with chronic pancreatitis, taking history of recurrent acute pancreatitis, medical imaging and biochemistry findings, lifestyle, and need for narcotic medications into account.5,6,16
A large proportion of people in our study reported that the pain and other features of hereditary pancreatitis impaired their daily activities and mental health. The fact that 38 people (86%) regularly used opioid medications is one indication of the severity of the pain caused by hereditary pancreatitis. The long term effects of opioid use and its social consequences are important for people with this condition.
Limitations
Ascertainment bias was possible in our study, and reporting of symptom onset and clinical features was subject to recall bias. We only included people with molecular diagnoses of hereditary pancreatitis, excluding those who had not undergone genetic testing, and also those who received molecular diagnoses outside the four participating services; consequently, we probably underestimated its prevalence. Further, inadequate clinical awareness of hereditary pancreatitis and its misdiagnosis as, for example, alcohol‐induced pancreatitis or opioid‐seeking behaviour, may have reduced its detection. Nonetheless, our study included the largest reported Australian sample of people with hereditary pancreatitis, and the number of patients was large compared with some overseas studies (eg, 38 people diagnosed in Denmark [population: 5.8 million] over a period of nearly 30 years21). We used whole exome sequencing to evaluate all known genetic causes of hereditary pancreatitis, and future studies using it as a comprehensive genetic tool alongside community case finding will determine whether the prevalence of hereditary pancreatitis is higher than we have reported.
Conclusion
We found the prevalence of hereditary pancreatitis in South Australia to be higher than reported in Europe, and it was markedly higher among Indigenous people than other South Australians. Building on our earlier reports,5,16 we have identified the number of South Australians with hereditary pancreatitis eligible for newer therapies, including TP‐IAT. Hereditary pancreatitis is an important but inadequately recognised cause of early onset pancreatitis in South Australia, particularly in young Indigenous people.
Open access
Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Box 1 – Hereditary pancreatitis pedigrees ascertained in South Australia, based on molecular diagnoses in 44 people, 1 January 2006 – 30 June 2021
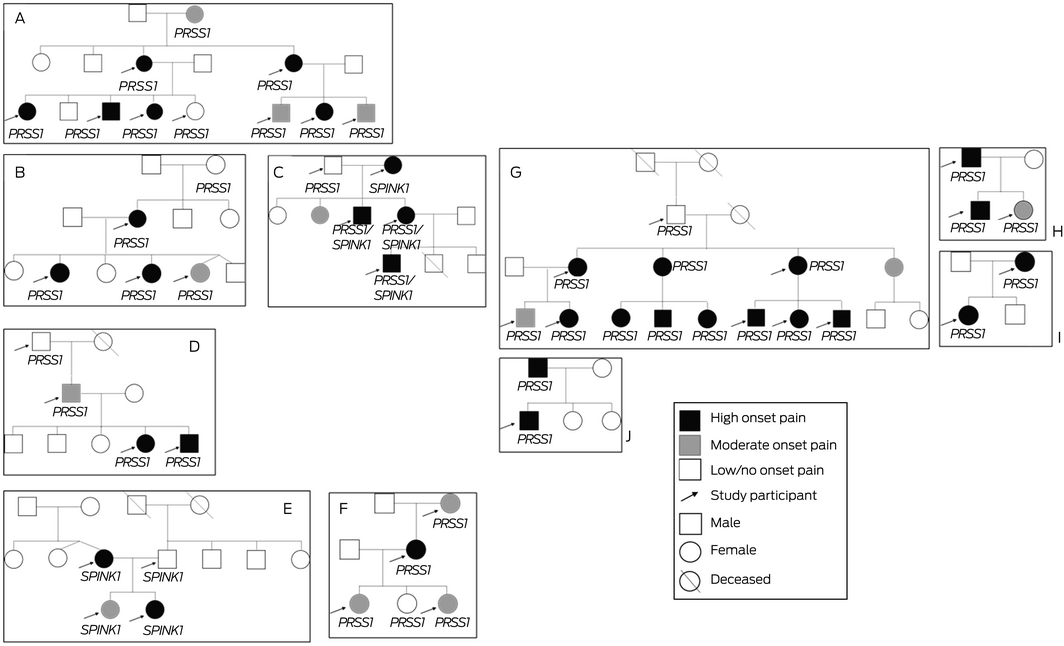
Mutations: family A: PRSS1 p.R122H; family B: PRSS1 p.N29I; family C: PRSS1 p.N29I, SPINK1 p.N34S; family D: PRSS1 p.N29I; family E: SPINK1 p.N34S; family F: PRSS1 p.R122H; family G: PRSS1 p.R122H; family H: PRSS1 p.N29I; family I: PRSS1, pN29I; family J: PRSS1 A16V.
Box 2 – Hereditary pancreatitis‐associated gene variants in 44 people diagnosed in South Australia, 1 January 2006 – 30 June 2021, and selected summary values from studies in Europe, France, and Denmark
|
|
South Australia, 2006–2021 |
1997–2004 |
2005–2008 |
1977–2004 |
|||||||||||
|
Gene/mutation |
Total number |
Indigenous Australians |
Europe19 |
France20 |
Denmark21 |
||||||||||
|
|
|||||||||||||||
|
Participants |
44 |
25 |
418 |
200 |
38 |
||||||||||
|
PRSS1 |
36 (82%) |
25 (100%) |
77% |
68% |
66% |
||||||||||
|
R122H |
21 (48%) |
17 (68%) |
52% |
53% |
37% |
||||||||||
|
N29I |
14 (32%) |
8 (32%) |
21% |
8% |
0 |
||||||||||
|
A16V |
1 (2%) |
0 |
4% |
NR |
29% |
||||||||||
|
SPINK1 |
|
|
|
|
|
||||||||||
|
N34S |
5 (11%) |
0 |
NR |
13% |
20% |
||||||||||
|
PRSS1/SPINK1 |
|
|
|
|
|
||||||||||
|
N29I/N34S |
3 (7%) |
0 |
NR |
NR |
NR |
||||||||||
|
CFTR |
0 |
0 |
NR |
2% |
11% |
||||||||||
|
CTRC |
0 |
0 |
NR |
NR |
NR |
||||||||||
|
CPA1 |
0 |
0 |
NR |
NR |
NR |
||||||||||
|
|
|||||||||||||||
|
NR = not reported. Genes: PRSS1 = cationic trypsinogen serine protease 1; SPINK1 = serine protease inhibitor Kazal‐type 1; CFTR = cystic fibrosis transmembrane conductance regulator; CTRC = chymotrypsin C; CPA1 = A‐type carboxypeptidase. |
|||||||||||||||
Box 3 – Clinical features of 44 people diagnosed with hereditary pancreatitis in South Australia, 1 January 2006 – 30 June 2021
|
Characteristic |
Number (proportion) |
||||||||||||||
|
|
|||||||||||||||
|
Age at time of study (years) |
|
||||||||||||||
|
< 10 |
6 (14%) |
||||||||||||||
|
11–20 |
18 (41%) |
||||||||||||||
|
21–40 |
7 (16%) |
||||||||||||||
|
More than 40 |
13 (30%) |
||||||||||||||
|
Age of onset (years) |
|
||||||||||||||
|
< 10 |
37 (84%) |
||||||||||||||
|
10–20 |
7 (16%) |
||||||||||||||
|
Smoking |
|
||||||||||||||
|
Never |
38 (86%) |
||||||||||||||
|
Past smoker |
4 (9%) |
||||||||||||||
|
Current smoker |
2 (5%) |
||||||||||||||
|
Alcohol use (past 12 months)* |
|
||||||||||||||
|
Never |
29 (66%) |
||||||||||||||
|
Light |
14 (32%) |
||||||||||||||
|
Moderate |
1 (2%) |
||||||||||||||
|
High |
0 |
||||||||||||||
|
Diabetes mellitus |
15 (34%) |
||||||||||||||
|
Pancreatic surgery |
8 (18%) |
||||||||||||||
|
Pseudocysts |
7 (16%) |
||||||||||||||
|
Cholelithiasis (gallstones) |
4 (9%) |
||||||||||||||
|
Satisfy Minnesota criteria† |
29 (66%) |
||||||||||||||
|
|
|||||||||||||||
|
* Light = fewer than five standard drinks/week; moderate = 5–10 standard drinks/week; heavy = more than ten standard drinks/week. † Assesses appropriateness of total pancreatectomy with islet auto‐transplantation for patients with chronic pancreatitis.6 |
|||||||||||||||
Box 4 – Quality of life for 44 people diagnosed with hereditary pancreatitis in South Australia, 2006–2021
|
Characteristic |
Number (proportion) |
||||||||||||||
|
|
|||||||||||||||
|
Frequency of attacks of pancreatitis (past 12 months)* |
|
||||||||||||||
|
None |
4 (9%) |
||||||||||||||
|
Low (fewer than five) |
8 (18%) |
||||||||||||||
|
Moderate (5–10) |
20 (45%) |
||||||||||||||
|
High (more than ten) |
12 (27%) |
||||||||||||||
|
Onset pain level (past 12 months)† |
|
||||||||||||||
|
Low/no |
5 (11%) |
||||||||||||||
|
Moderate |
10 (23%) |
||||||||||||||
|
High |
29 (66%) |
||||||||||||||
|
Weight loss (past 12 months) |
14 (32%) |
||||||||||||||
|
Regularly use opioid medications |
38 (86%) |
||||||||||||||
|
Self‐reported quality of life (past six months)‡ |
|
||||||||||||||
|
Low |
20 (45%) |
||||||||||||||
|
Moderate |
24 (55%) |
||||||||||||||
|
High |
0 |
||||||||||||||
|
Impact of pain on physical wellbeing (past four weeks)§ |
|
||||||||||||||
|
None |
4 (9%) |
||||||||||||||
|
Light |
5 (11%) |
||||||||||||||
|
Moderate |
16 (36%) |
||||||||||||||
|
High |
19 (43%) |
||||||||||||||
|
Impact of pain on mental health (past four weeks)¶ |
|
||||||||||||||
|
None |
2 (5%) |
||||||||||||||
|
Light |
24 (55%) |
||||||||||||||
|
Moderate |
13 (30%) |
||||||||||||||
|
High |
5 (11%) |
||||||||||||||
|
|
|||||||||||||||
|
* Self‐resolving or leading to hospital admission. † Low = no interference with activity, did not require narcotic medication; moderate = moderate interference with activity, immediate need for pain management but not hospitalisation or medical help; high = high interference with activity, immediate need for pain management and needed hospitalisation or medical help. ‡ Low = daily activity largely hindered by hereditary pancreatitis; moderate = daily activity moderately affected by hereditary pancreatitis; high = daily activity not affected by hereditary pancreatitis. § Based on responses to physical health and accomplishment of daily tasks in standardised 12‐item Short Form Health Survey (SF‐12). ¶ Based on responses to SF‐12 items about perceived changes in levels of calmness, peacefulness, energy, and down‐heartedness because of hereditary pancreatitis. |
|||||||||||||||
Received 9 August 2021, accepted 7 January 2022
- Denghao Wu1
- Tristan J Bampton2,3
- Hamish S Scott4
- Alex Brown5,6
- Karin Kassahn1,4
- Christopher Drogemuller1,3
- Sunita MC De Sousa1,3
- David Moore7
- Thuong Ha4
- John WC Chen8
- Sanjeev Khurana7
- David J Torpy3
- Toni Radford3
- Richard Couper7
- Lyle Palmer2
- P Toby Coates1,3
- 1 Adelaide Medical School, University of Adelaide, Adelaide, SA
- 2 The University of Adelaide, Adelaide, SA
- 3 Royal Adelaide Hospital, Adelaide, SA
- 4 SA Pathology, Adelaide, SA
- 5 Aboriginal Health Research, South Australian Health and Medical Research Institute, Adelaide, SA
- 6 University of South Australia, Adelaide, SA
- 7 Women's and Children's Hospital Adelaide, Adelaide, SA
- 8 Flinders Medical Centre, Adelaide, SA
We thank the Royal Adelaide Hospital Research Foundation for funding whole exome sequencing research (2020 RRC CPG 12862) and for providing research grant funding to Denghao Wu (s‐05‐misc‐2020).
No relevant disclosures.
- 1. Rebours V, Lévy P, Ruszniewski P. An overview of hereditary pancreatitis. Dig Liver Dis 2012; 44: 8‐15.
- 2. Raphael KL, Willingham FF. Hereditary pancreatitis: current perspectives. Clin Exp Gastroenterol 2016; 9: 197‐207.
- 3. Kleeff J, Whitcomb DC, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers 2017; 3: 17060.
- 4. Lévy P, Domínguez‐Muñoz JE, Imrie C, et al. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United Eur Gastroent 2014; 2: 345‐354.
- 5. Eldredge J, Couper MR, Moore DJ, et al. South Australian experience with paediatric total pancreatectomy and islet autotransplantation for PRSS1‐associated hereditary pancreatitis. Med J Aust 2021; 215: 294‐296.e1. https://www.mja.com.au/journal/2021/215/7/south‐australian‐experience‐paediatric‐total‐pancreatectomy‐and‐islet
- 6. Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology 2014; 14: 27‐35.
- 7. Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996; 14: 141‐145.
- 8. Shelton CA, Umapathy C, Stello K, et al. Hereditary pancreatitis in the United States: survival and rates of pancreatic cancer. Am J Gastroenterol 2018; 113: 1376.
- 9. Weiss F. Pancreatic cancer risk in hereditary pancreatitis. Front Physiol 2014; 5: 70.
- 10. Rosendahl J, Bödeker, Mössner JH, Teich N. Hereditary chronic pancreatitis. Orphanet J Rare Dis 2007; 2: 1.
- 11. Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet 2013; 45: 1216‐1220.
- 12. Felderbauer P, Klein W, Bulut K, et al. Mutations in the calcium‐sensing receptor: a new genetic risk factor for chronic pancreatitis? Scand J Gastroenterol 2006; 41: 343‐348.
- 13. Kumar S, Ooi CY, Werlin S, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr 2016; 170: 562‐569.
- 14. Liu QY, Abu‐El‐Haija M, Husain SZ, et al. Risk factors for rapid progression from acute recurrent to chronic pancreatitis in children: report from INSPPIRE. J Pediatr Gastroenterol Nutr 2019; 69: 206‐211.
- 15. Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 2010; 7: 131‐145.
- 16. Bampton TJ, Holmes‐Walker DJ, Drogemuller CJ, et al; Australian Islet Consortium. Australian experience with total pancreatectomy with islet autotransplantation to treat chronic pancreatitis. ANZ J Surg 2021; 91: 2663‐2668.
- 17. Ware J, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220‐333.
- 18. Schneider M, Radbone CG, Vasquez SA, et al. Population data centre profile: SA NT DataLink (South Australia and Northern Territory). Int J Popul Data Sci 2019; 4: 1136.
- 19. Howes N, Lerch MM, Greenhalf W, et al; European Registry of Hereditary Pancreatitis and Pancreatic Cancer (EUROPAC). Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2: 252‐261.
- 20. Rebours V, Boutron‐Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut 2009; 58: 97‐103.
- 21. Joergensen MT, Brusgaard K, Crüger DG, et al. Genetic, epidemiological, and clinical aspects of hereditary pancreatitis: a population‐based cohort study in Denmark. Am J Gastroenterol 2010; 105: 1876‐1883.
- 22. Australian Bureau of Statistics. National, state and territory population; reference period December 2020. Updated 17 June 2021. https://www.abs.gov.au/statistics/people/population/national‐state‐and‐territory‐population/dec‐2020 (viewed June 2021).
- 23. Australian Bureau of Statistics. Census of population and housing. Reflecting Australia: stories from the census, 2016. Aboriginal and Torres Strait Islander population. June 2017. https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/2071.0main+features102016 (viewed July 2021).
- 24. Cervantes A, Waymouth EK, Petrov MS. African‐Americans and indigenous peoples have increased burden of diseases of the exocrine pancreas: a systematic review and meta‐analysis. Dig Dis Sci 2019; 64: 249‐261.
- 25. McGaughran JM, Kimble R, Upton J, George P. Hereditary pancreatitis in a family of Aboriginal descent. J Paediatr Child Health 2004; 40: 487‐489.






Abstract
Objective: To characterise the clinical phenotypes and genetic variants of hereditary pancreatitis in people diagnosed in South Australia.
Design, setting, participants: Cross‐sectional study of people who received molecular diagnoses of hereditary pancreatitis from one of four major diagnostic services in South Australia, 1 January 2006 – 30 June 2021.
Main outcome measures: Genotypic and clinical features of people with hereditary pancreatitis, including age at onset, attack frequency, pain indices, use of opioid medications, and physical and mental health impact of hereditary pancreatitis.
Results: We identified 44 people from ten families who received molecular diagnoses of hereditary pancreatitis during 2006–21 (including 25 Indigenous people [57%] and 27 women [61%]): 36 withPRSS1 , five with SPINK1 , and three with PRSS1 and SPINK1 mutations (determined by whole exome sequencing). Symptom onset before the age of ten years was reported by 37 people (84%). Pancreatitis‐related pain during the preceding four weeks was described as moderate or high by 35 people (79%); 38 people regularly used opioids (86%). Fifteen patients had diabetes mellitus (34%), and eight had undergone pancreatic surgery (18%). The estimated prevalence of hereditary pancreatitis was 1.1 (95% CI, 0.72–1.4) cases per 100 000 population for non‐Indigenous and 71 (95% CI, 66–77) cases per 100 000 population for Indigenous South Australians. Among people with adult‐onset chronic pancreatitis admitted to South Australian public hospitals during 2001–2019, the proportions of Indigenous people (12%) and women (38%) were smaller than we report for hereditary pancreatitis.
Conclusion: The estimated prevalence of hereditary pancreatitis in South Australia is higher than in Europe.PRSS1 gene mutations are important causes, particularly among Indigenous young people.