The known: No recent population‐based studies of factors associated with different types of treatment for men with prostate cancer have been undertaken in New South Wales.
The new: Men in NSW aged 45 years or more with prostate cancer were twice as likely to undergo radical prostatectomy as external beam radiotherapy, and few had consulted radiation oncologists prior to surgery. The treatment type received was influenced by socio‐demographic factors, including having private health insurance.
The implications: Given the differing side effects and costs of the treatment options, health policies regarding the treatment of prostate cancer should be re‐examined.
Prostate cancer is the most frequently diagnosed cancer in Australian men; about 19 508 cases were diagnosed in 2019.1 Treatment options include surgery, radiotherapy, and active surveillance. Survival rates for men with localised prostate cancer are similar with all options, but the side effects profiles differ markedly, including higher rates of urinary incontinence and sexual dysfunction after surgery and of rectal bleeding after radiotherapy.2,3
Population‐based studies of patterns of care in Victoria and South Australia (2016)4 and Victoria alone (2020)5 have been published, but the only recent population‐based study of factors associated with prostate cancer treatment types in New South Wales investigated the treatment of Aboriginal men.6 Further, the factors that determine the type of treatment received by men with prostate cancer, and the consequent costs, have been subjects of controversy.7,8 The aims of our study were therefore to describe the patterns of care for men in the NSW 45 and Up Study with prostate cancer, and to ascertain the factors associated with receiving different types of treatment.
Methods
A total of 267 153 NSW men and women aged 45 years or more were enrolled in the 45 and Up Study of the Sax Institute, Sydney, during January 2006 – December 2009.9 The 45 and Up Study is a large cohort study of people aged 45 or more investigating exposures and outcomes of public health importance for older people. Prospective participants were randomly selected from the Services Australia (formerly the Australian Department of Human Services) enrolment database. Enrolled participants completed a postal baseline questionnaire comprising 58 questions about health, lifestyle, and socio‐demographic characteristics and consented to linkage of their data with administrative health datasets.
Data collection and linkage
The record linkage undertaken has been described previously.10 The datasets linked to the 45 and Up Study baseline questionnaire data were:
- Medicare Benefits Schedule (MBS) claims, to June 2016;
- Pharmaceutical Benefits Scheme (PBS) claims, to June 2016;
- the NSW Cancer Registry, to December 2013;
- the NSW Admitted Patient Data Collection (APDC; public and private hospital inpatient services), to June 2016; and
- the NSW Register of Births, Deaths and Marriages, for deaths to June 2016.
The MBS and PBS datasets were linked to the baseline questionnaire data using a unique identifier supplied to Services Australia; the other three datasets were probabilistically linked by the Centre for Health Record Linkage using a method that preserved anonymity.11
45 and Up Study participants with incident primary prostate cancers diagnosed after completing the baseline questionnaire were identified in the NSW Cancer Registry. We excluded patients who held Department of Veterans’ Affairs health cards, as their MBS/PBS claims data would be incomplete. Patients were also excluded if they had more than one incident cancer or had previously undergone radiotherapy, if the month of diagnosis was unknown, or if they had been diagnosed from their death certificate.
Patients were deemed to have received radical prostatectomy, external beam radiotherapy (EBRT), high or low dose rate brachytherapy if corresponding MBS or APDC items were recorded after the diagnosis date; to have received chemotherapy if corresponding MBS, APDC or PBS items were recorded after the diagnosis date; and to have received androgen deprivation therapy if corresponding PBS items were recorded after the diagnosis date. Patients without recorded items for EBRT, radical prostatectomy, high or low dose brachytherapy, chemotherapy, or androgen deprivation therapy were deemed to have received no active treatment.
Patients were categorised as having radiation oncologist consultations if MBS items for either radiation oncology specialist or radiation oncology consultant physician attendances were recorded.
Socio‐demographic, socio‐economic, and health characteristics
The period of observation for each patient was the time from prostate cancer diagnosis to 30 June 2016. Socio‐demographic and socio‐economic data12 were derived from the baseline questionnaire responses. “Patients without private health insurance” included those who held health care cards or had Medicare coverage only. Area‐level socio‐economic status (Index of Relative Socioeconomic Disadvantage13) was based on participants’ place of residence. Functional limitations were assessed in the baseline questionnaire with the Medical Outcomes Study (MOS) SF‐36 physical functioning scale (PF10; score of 100 = no limitations).14 Comorbidity data (Charlson comorbidity index) were derived from diagnosis codes recorded in the APDC dataset, as previously described;12 data for other health factors were derived from the baseline questionnaire responses.
Statistical analysis
Adjusted subdistribution hazard ratios for receiving radical prostatectomy and radiotherapy were separately estimated (with 95% confidence intervals [CIs]) by competing risks survival regression, with death from any cause as the competing risk and including age at diagnosis, cancer stage, and each socio‐demographic, socio‐economic and health characteristic in turn.15 Multivariable regression with purposeful variable selection was then undertaken by adding statistically significant socio‐demographic, socio‐economic and health variables, together with age and cancer stage. Proportional hazards assumptions were examined. Missing data were managed as described previously.12 For variables with large numbers of missing responses (“need help with daily tasks”, body mass index), a separate “missing” category was included. For other variables, missing responses were not included in the analysis.
Analyses were performed in SAS 9.4 or Stata 16.0.
Ethics approval
The 45 and Up Study was approved by the University of New South Wales Human Research Ethics Committee (reference, 10186) and the NSW Population and Health Services Research Ethics Committee (reference, 2014/08/551).
Results
After record linkage and exclusions, 4003 men with new prostate cancer diagnoses during 2006–2013 were included in our study (Box 1); 641 patients with prostate cancer (13.8%) were excluded because they held Department of Veterans’ Affairs health cards health cards. Median follow‐up time since diagnosis was 5.4 years (interquartile range [IQR], 3.9–6.8 years). The median age of participants was 68 years (IQR, 62–75 years); 1588 (40%) were aged 60–69 years, and 2207 (55%) had localised cancers (Box 2).
Treatment
In total, 1619 of 4003 patients (40%) underwent radical prostatectomy, including 1036 of 2207 men with localised cancer (47%). EBRT was received by 893 patients (22%), including 433 with localised cancer (20%); 183 received low or high dose rate brachytherapy (5%), 87 chemotherapy (2%), 373 androgen deprivation therapy alone (9%), and 848 no active treatment (21%) (Box 3). Of the 1466 patients with APDC codes for radical prostatectomy, 1117 underwent radical prostatectomy in private hospitals (76%) and 349 in public hospitals (24%).
Radiation oncology consultation
Of the 1787 patients who had radiation oncology consultations (45%), 1298 had EBRT (72%) and 98 low dose rate brachytherapy only (5%). Of 1628 patients who had radical prostatectomy alone or with other treatments, 535 had radiation oncology consultations at some point (33%), including 205 prior to radical prostatectomy (13%). For the 1041 patients with localised disease who underwent radical prostatectomy alone or together with other treatments, 287 had radiation oncology consultations (28%), including 129 prior to radical prostatectomy (12%).
Factors associated with type of treatment
Multivariable regression analyses for undergoing radical prostatectomy or EBRT, adjusted for age and stage of disease, are summarised in table 1 in the online Supporting Information.
In the full multivariable analysis, factors associated with greater likelihood of radical prostatectomy were age 45–59 years, regional stage disease, living 100 km or more from the nearest radiotherapy centre, being partnered or married, and having private health insurance; lower physical functioning (PF10 score below 90), obesity, and living in areas of greater socio‐economic disadvantage (Index of Relative Socioeconomic Disadvantage quintiles 3 and 5) were associated with lower likelihood of radical prostatectomy (Box 4).
In the full multivariable analysis, factors associated with greater likelihood of EBRT were age 70–79 years, non‐localised or unknown stage disease, living less than 100 km from the nearest radiotherapy centre, and not having private health insurance. Age 45–59 years or greater than 80 years, and having two or more comorbid conditions were each associated with lower likelihood of EBRT (Box 5).
We identified minor violations of the proportional hazards assumptions, and therefore conducted multivariable analyses for each outcome at three and 12 months’ follow‐up. The results of these analyses did not markedly differ from those of our major analyses (Supporting Information, tables 2 and 3).
Discussion
Among 4003 NSW men aged 45 years or more who were diagnosed with prostate cancer during 2006–2013, the proportion who had radical prostatectomies was almost twice as large as that of patients who received EBRT for primary treatment, and the difference was even more marked for patients with localised disease. Similar proportions underwent radical prostatectomy and EBRT in earlier Australian studies.4,5 In contrast, EBRT was twice as common as radical prostatectomy in a British study,16 while the use of radical prostatectomy was similar to that of EBRT or brachytherapy in a recent American study.17 The differences between Australia and these countries may be partly explained by differences in their health systems and costs reimbursement schedules. In the United Kingdom, most radical prostatectomies and EBRT are delivered by the National Health Service (NHS),16 which provides uro‐oncology clinics in which patients are seen by both urologists and radiation oncologists,18 and there are no financial incentives for treating doctors to recommend particular treatments. In the United States, the use of intensity‐modulated radiotherapy (a form of EBRT) has increased; this may be partly because urologists can own practices offering this treatment, to which they can refer their own patients.19 In Australia, most radical prostatectomies are performed in private hospitals, and patients incur out‐of‐pocket costs, whereas most EBRT is undertaken in public hospitals, at no cost to the patient.
Although survival rates after radical prostatectomy and EBRT are similar,2 their side effects profiles differ markedly.3,20 Rates of patient‐reported urinary incontinence requiring pads are higher six years after radical prostatectomy than after EBRT (17% v 4%) and potency rates lower (17% v 27%); the rate of bloody stools is higher after EBRT (6% v 1%).3 EBRT has also been associated with higher rates of hospitalisation and second malignancies.20 However, EBRT is less invasive than radical prostatectomy, and can be performed as an outpatient procedure without anaesthesia. Side effects of treatment for prostate cancer can be associated with decision regret.21
Given the impact these differences may have on quality of life, patients should be informed about all treatment options. However, only 13% of patients in our study who had radical prostatectomies had had radiation oncology consultations before the operation. This suggests that most patients who have radical prostatectomies do so without being advised by radiation oncologists about EBRT, an equally effective alternative. A recent NSW study found that deciding between radical prostatectomy and EBRT by prostate cancer patients largely depended on their urologists’ recommendations.22 In addition, patients erroneously believe that radical prostatectomy provides a greater chance of cure than EBRT.22 A Canadian study found that patients who saw both a urologist and a radiation oncologist were more likely to undergo radiotherapy than those who saw only a urologist.23
We found that having private health insurance was associated with undergoing radical prostatectomy, and patients without insurance were more likely to receive EBRT. Three‐quarters of patients who had radical prostatectomies did so in private hospitals. Patients in Australia receive private health insurance rebates for radical prostatectomy, but not for EBRT. Having private health insurance and being able to bear out‐of‐pocket costs may therefore influence the type of treatment received. Our finding that patients from socio‐economically more disadvantaged areas were less likely to have radical prostatectomies is consistent with this view. Having private health insurance has been associated with higher out‐of‐pocket costs for Australian patients with prostate cancer.8 A 2005 study in Western Australia found that men admitted to private hospitals were twice as likely to undergo radical prostatectomy as men treated in public hospitals.24 A UK study similarly found that patients diagnosed in private hospitals were more likely to undergo radical prostatectomy in preference to EBRT than those diagnosed in the NHS.25 Increasing patients’ access to radical prostatectomy in the NSW public health system could remove some of the socio‐economic barriers to receiving this treatment.
Several other characteristics were found to influence the type of treatment received. Patients with partners were more likely to have radical prostatectomies, as also reported by an American study;26 the perceptions of the patient and their partners or caregivers of the benefits and risks of radical prostatectomy for men with partners may be important. Patients who lived 100 km or more from a radiotherapy centre were less likely to receive EBRT, suggesting access problems. Patients who worked fulltime were also less likely to undergo EBRT, probably because traditional EBRT regimens typically require daily treatment over nine weeks. The development of shorter stereotactic treatments delivered over one or two weeks may reduce the logistic problems for fulltime employees considering EBRT. Finally, we found that age, number of comorbid conditions, body mass index, and level of physical functioning each influenced the type of treatment received by patients, possibly reflecting their general fitness for treatment.
Limitations
One limitation of our study was the lack of clinical detail in the linked data, including TNM category and Gleason score; we were consequently unable to examine treatment patterns for specific risk groups. We were also unable to determine which patients not receiving active treatment were on active surveillance (close monitoring with regular prostate‐specific antigen tests or biopsies) or watchful waiting (management predominantly according to symptoms). The proportion of men with localised cancer not receiving active treatment (20%) was lower than in some other reports (low risk cancers, 44%);4 the reasons for the difference are unclear. We analysed data from the 45 and Up Study, in which the participation rate was about 18%;9 the proportions of people aged 80 years or more, people living in rural or remote areas, and people with higher incomes were higher than in the general NSW population. However, it has been reported that, despite some differences in outcomes from another NSW study with a higher response rate, the exposure–outcome relationships were similar.27 Further, we excluded the 13.8% of patients with prostate cancer with Department of Veterans’ Affairs health cards, who were of higher median age than the patients included in our study; further, a larger proportion lived in higher socio‐economic status areas, and all have private insurance (data not shown). One‐third of patients in our study had been diagnosed with prostate cancer before 2010; however, we found no association between year of diagnosis and type of treatment received, suggesting that our findings based on 4003 patients are relevant to clinical practice. Socio‐demographic and health characteristics data were based on baseline questionnaire responses, and we could not adjust for changes in these characteristics by the time of prostate cancer diagnosis. Finally, we were unable to account for patient preference, which also influences the type of treatment received.
Conclusion
We identified several socio‐demographic and socio‐economic characteristics that may influence the type of treatment received by men with prostate cancer in NSW. This is an important public health question, given the potential harms of treatment, including long term side effects3,20,21 and the financial costs.8 The Prostate Cancer Outcomes Registry of Australia and New Zealand (PCOR‐ANZ) is rapidly becoming a useful source of population‐wide clinical, treatment, and outcomes data.28 Linking the data sources we used with the PCOR‐ANZ could help further advance our knowledge of the consequences of the referral patterns we have described, including for patient‐reported quality of life. The restricted access of patients in rural and remote regions to radiotherapy should be remediated by appropriate cancer services planning, telemedicine, and assistance with transport and accommodation. Our findings also suggest that patients do not typically consult both urologists and radiation oncologists about treatment choices; several uro‐oncology specialist clinics have been established in Australia to help overcome this problem.22 We need education programs to ensure that patients, their partners and caregivers, and their physicians are fully informed about all treatment options. Finally, associations between socio‐economic factors and treatment received suggest the need to review policies that affect access to the different treatment options for men with prostate cancer in Australia.
Box 1 – Selection of men in the 45 and Up Study with incident prostate cancer diagnosed 2006–2013
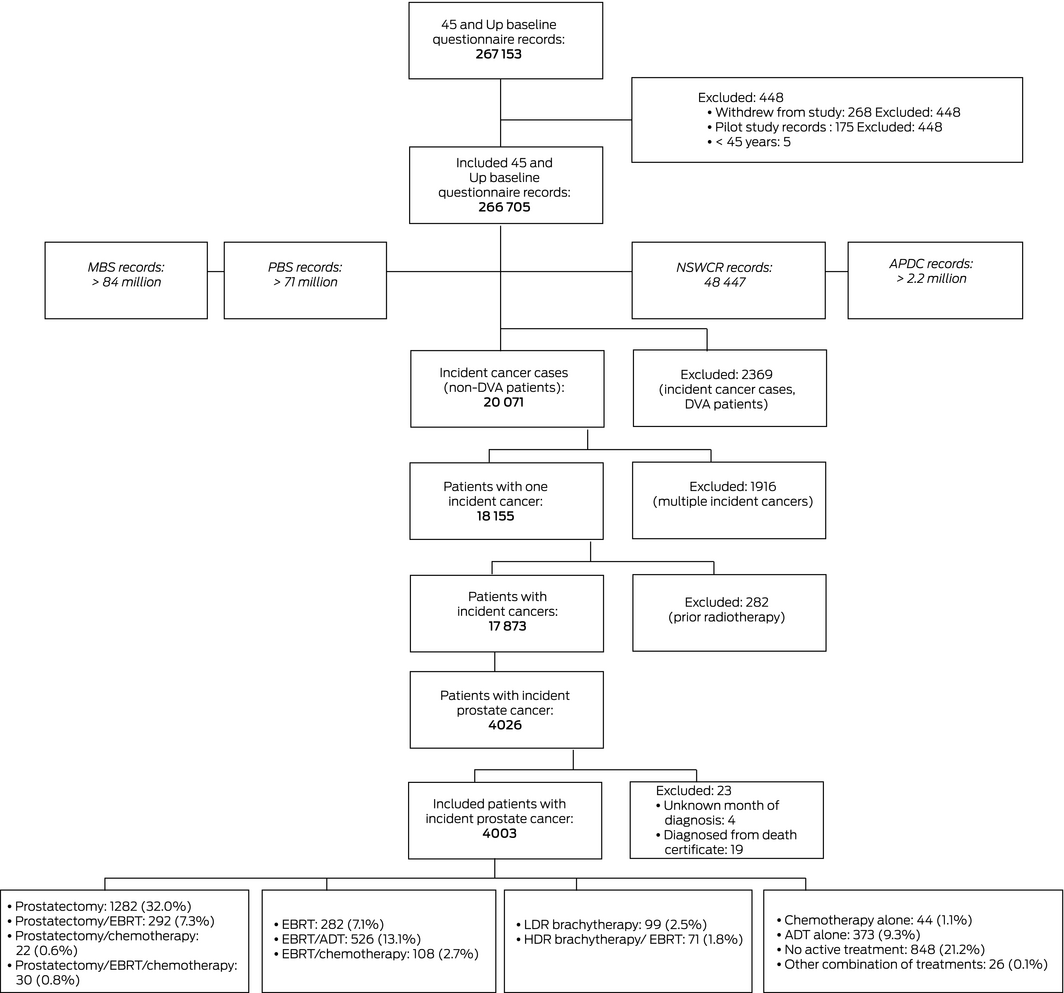
ARIA = Accessibility Remoteness Index of Australia;29 ADT = androgen deprivation therapy; APDC = New South Wales Admitted Patient Data Collection; DVA = Department of Veterans’ Affairs; EBRT = external beam radiotherapy; HDR = high dose rate; LDR = low dose rate; MBS = Medicare Benefits Schedule; NSWCR = New South Wales Cancer Registry; PBS = Pharmaceutical Benefits Scheme.
Box 2 – Characteristics at diagnosis for 4003 men diagnosed in New South Wales with incident prostate cancer, 2006–2013
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Stage |
|
||||||||||||||
|
Localised |
2207 (55%) |
||||||||||||||
|
Regional |
410 (10%) |
||||||||||||||
|
Distant |
115 (3%) |
||||||||||||||
|
Unknown |
1271 (32%) |
||||||||||||||
|
Age (years) |
|
||||||||||||||
|
45–59 |
656 (16%) |
||||||||||||||
|
60–69 |
1588 (40%) |
||||||||||||||
|
70–79 |
1223 (31%) |
||||||||||||||
|
80 or more |
536 (13%) |
||||||||||||||
|
Year of diagnosis |
|
||||||||||||||
|
2006 |
71 (2%) |
||||||||||||||
|
2007 |
105 (3%) |
||||||||||||||
|
2008 |
409 (10%) |
||||||||||||||
|
2009 |
715 (18%) |
||||||||||||||
|
2010 |
652 (16%) |
||||||||||||||
|
2011 |
679 (17%) |
||||||||||||||
|
2012 |
683 (17%) |
||||||||||||||
|
2013 |
689 (17%) |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – First active treatment of men diagnosed in New South Wales with incident prostate cancer, 2006–2013
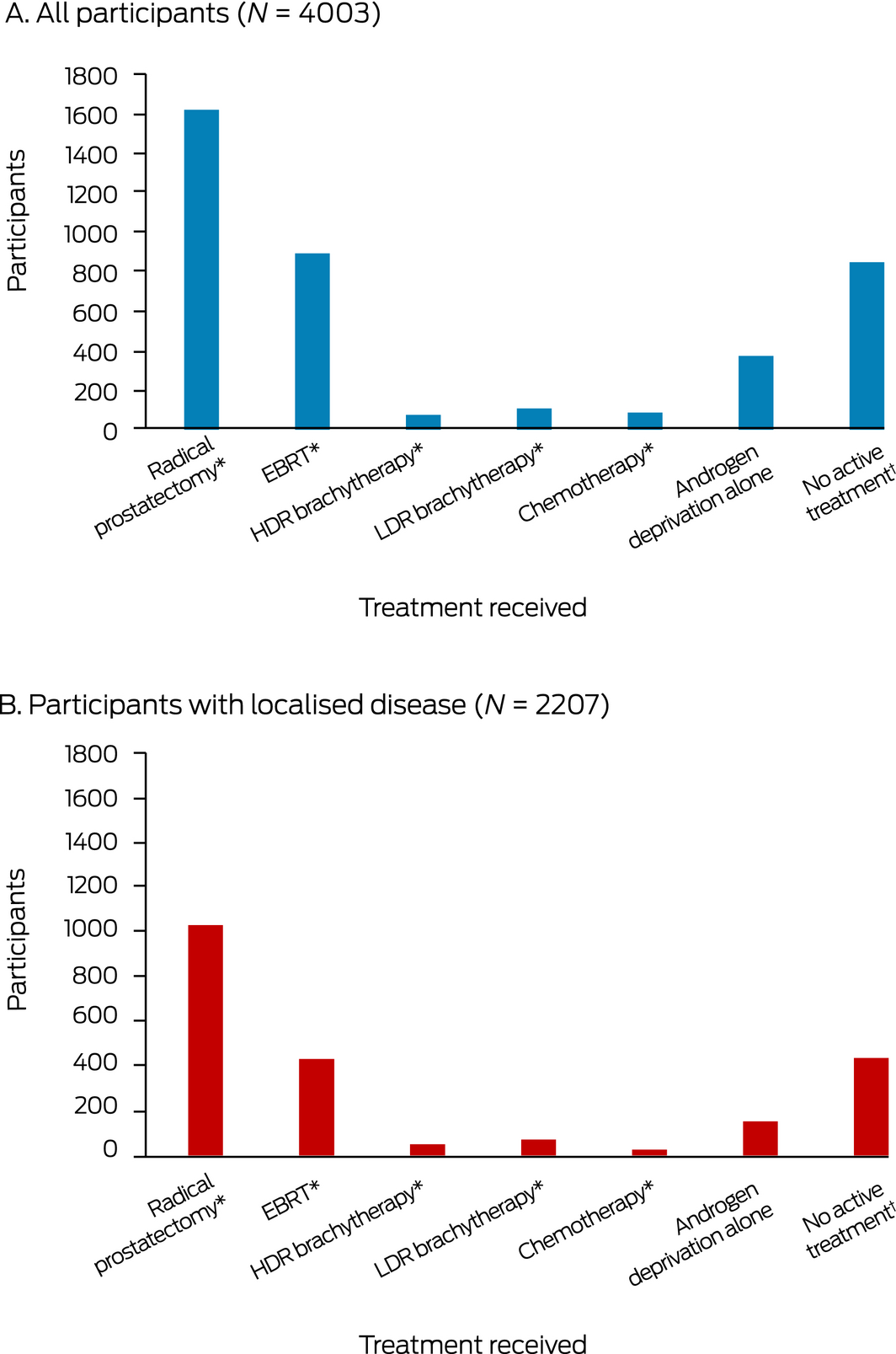
EBRT = external beam radiotherapy; HDR = high dose rate; LDR = low dose rate. * Patients who were treated with prostatectomy, EBRT, brachytherapy, or chemotherapy may have also used neoadjuvant androgen deprivation therapy. † Includes patients under active surveillance.
Box 4 – Characteristics associated with undergoing radical prostatectomy for prostate cancer diagnosed in New South Wales, 2006–2013 (N = 3774): multivariable analysis, fully adjusted*
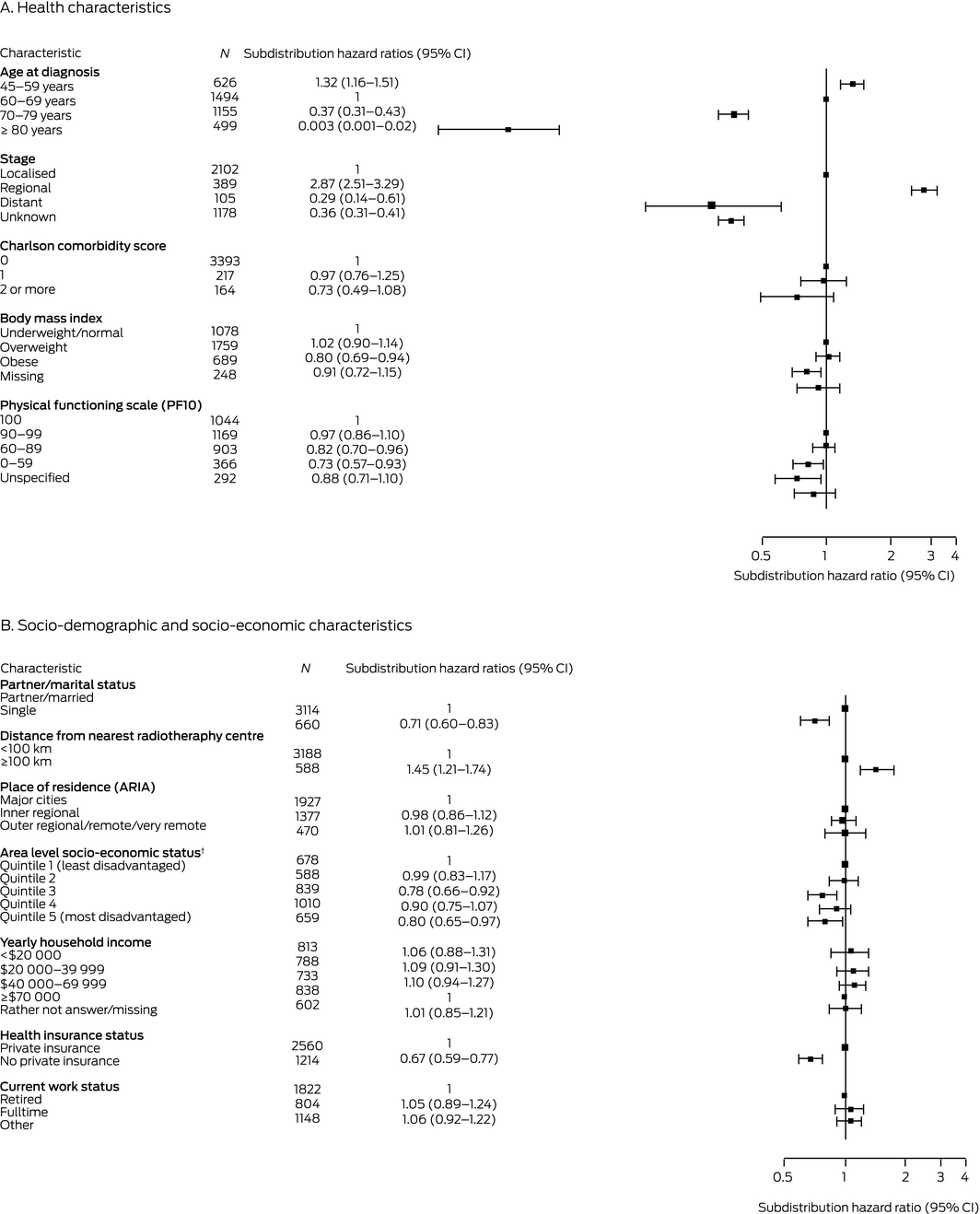
ARIA = Accessibility Remoteness Index of Australia;29 CI = confidence interval. * Adjusted for all other health, socio‐demographic, and socio‐economic factors; data for 229 men were not included because of missing data for one or more variables without a missing data category. † Socio‐economic Indexes for Areas (SEIFA), Index of Relative Socioeconomic Disadvantage 2006.13
Box 5 – Characteristics associated with receiving external beam radiotherapy for prostate cancer diagnosed in New South Wales, 2006–2013 (N = 3774): multivariable analysis, fully adjusted*
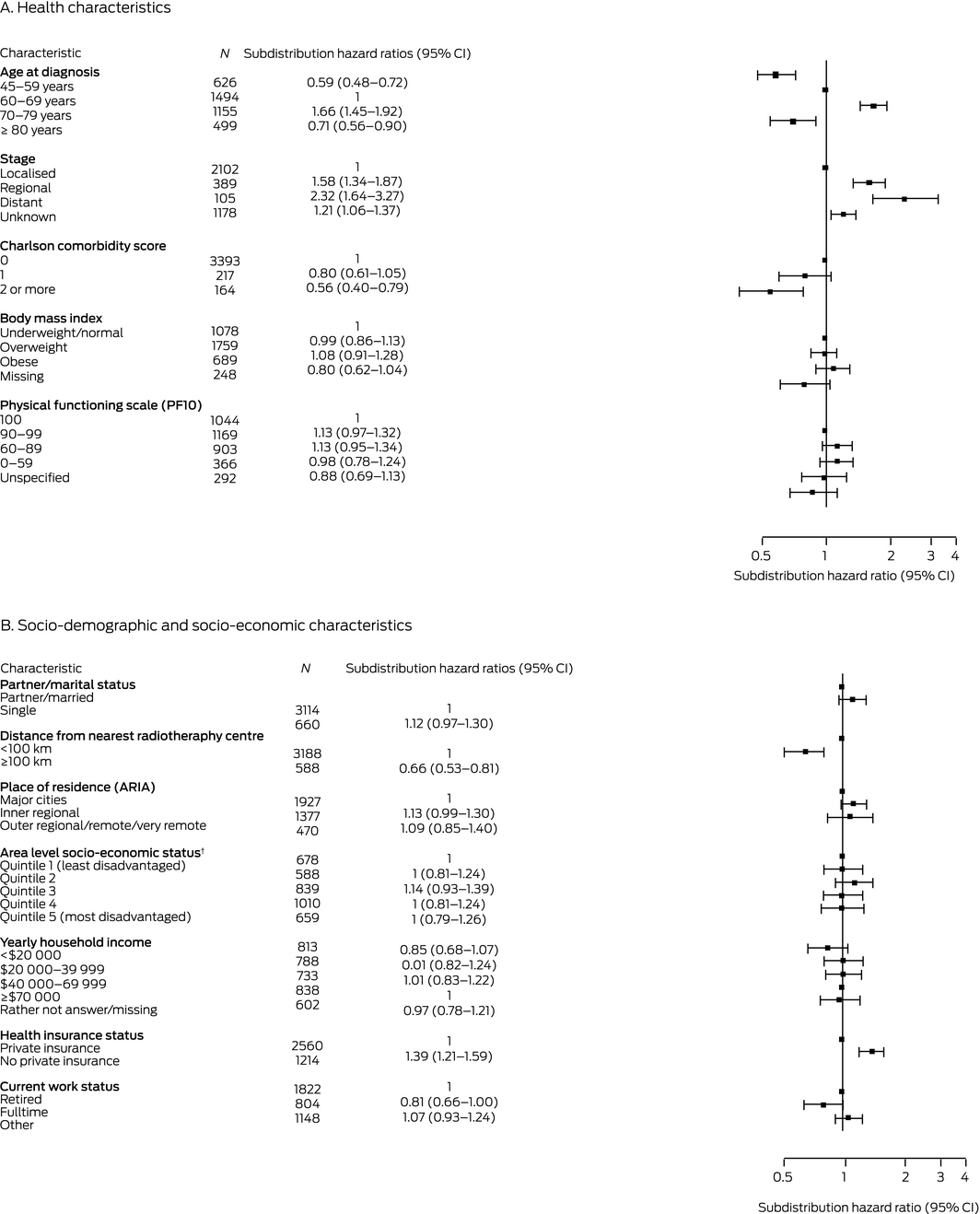
ARIA = Accessibility Remoteness Index of Australia;29 CI = confidence interval. * Adjusted for all other health, socio‐demographic, and socio‐economic factors; data for 229 men were not included because of missing data for one or more variables without a missing data category. † Socio‐economic Indexes for Areas (SEIFA), Index of Relative Socioeconomic Disadvantage 2006.13
Received 2 May 2020, accepted 7 October 2020
- Mei Ling Yap1,2,3
- Dianne L O'Connell2,3
- David E Goldsbury2,3
- Marianne F Weber2,3
- David P Smith2,3
- Michael B Barton1
- 1 Collaboration for Cancer Outcomes, Research and Evaluation (CCORE), Ingham Institute, University of New South Wales, Sydney, NSW
- 2 Cancer Council NSW, Sydney, NSW
- 3 University of Sydney, Sydney, NSW
We analysed data collected for the 45 and Up Study (https://www.saxinstitute.org.au/our-work/45-up-study). The 45 and Up Study is managed by the Sax Institute in collaboration with its major partner, Cancer Council NSW; other partners are the National Heart Foundation of Australia (NSW Division); the NSW Ministry of Health; NSW Government Family and Community Services/Ageing, Carers and the Disability Council NSW; and Australia Red Cross Blood Services. We thank the many thousands of people participating in the 45 and Up Study, the Centre for Health Record Linkage for linking the data, and the data custodians (NSW Health, NSW Cancer Institute, NSW Registry of Births, Deaths and Marriages, and Services Australia) for making the data available. We also thank Gabriel Gabriel (Collaboration for Cancer Outcomes, Research and Evaluation, Ingham Institute) and Sarsha Yap (Cancer Council NSW) for their advice on statistical methods.
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Cancer in Australia 2019 (Cancer series no.119; Cat. no. CAN 123). Canberra: AIHW, 2019.
- 2. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10‐Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–1424.
- 3. Donovan JL, Hamdy FC, Lane JA, et al; ProtecT Study Group. Patient‐reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016; 375: 1425–1437.
- 4. Ruseckaite R, Beckmann K, O’Callaghan M, et al. A retrospective analysis of Victorian and South Australian clinical registries for prostate cancer: trends in clinical presentation and management of the disease. BMC Cancer 2016; 16: 607.
- 5. te Marvelde L, Milne RL, Hornby CJ, et al. Differences in treatment choices for localised prostate cancer diagnosed in private and public health services. Med J Aust 2020; 213: 411–417. https://www.mja.com.au/journal/2020/213/9/differences-treatment-choices-localised-prostate-cancer-diagnosed-private-and
- 6. Rodger JC, Supramaniam R, Gibberd AJ, et al. Prostate cancer mortality outcomes and patterns of primary treatment for Aboriginal men in New South Wales, Australia. BJU Int 2015; 115 (Suppl 5): 16–23.
- 7. Haines I. The scandal of prostate cancer management in Australia. InSight+, 21 Nov 2016. https://insightplus.mja.com.au/2016/45/the-scandal-of‐prostate-cancer-management-in-australia (viewed Feb 2021).
- 8. Gordon LG, Walker SM, Mervin MC, et al. Financial toxicity: a potential side effect of prostate cancer treatment among Australian men. Eur J Cancer Care (Engl) 2017; 26: e12392.
- 9. 45 and Up Study Collaborators; Banks E, Redman S, et al. Cohort profile: the 45 and Up Study. Int J Epidemiol 2008; 37: 941‐947.
- 10. Yap ML, O’Connell D, Goldsbury D, et al. Comparison of four methods for estimating actual radiotherapy utilisation using the 45 and Up Study cohort in New South Wales, Australia. Radiother Oncol 2019; 131: 14–20.
- 11. Centre for Health Record Linkage. Master linkage key (MLK). http://www.cherel.org.au/master-linkage-key (viewed Feb 2020).
- 12. Yap ML, O’Connell D, Goldsbury D, et al. Factors associated with radiotherapy utilisation in New South Wales, Australia: results from the 45 and Up Study. Clin Oncol (R Coll Radiol) 2020; 32: 282–291.
- 13. Pink B. An introduction to socio‐economic indexes for areas (SEIFA). 2006 (ABS Cat. no. 2039.0). Canberra: Australian Bureau of Statistics, 2008. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2039.02006 (viewed Feb 2021).
- 14. Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483.
- 15. Fine JP, Grey RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
- 16. Turo R, Bromage S, Smolski M, et al. The changes in prostate cancer and its management in the North West of England over a 10‐year period. J Clin Urol 2015; 8: 315–320.
- 17. Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol 2018; 3: 170–180.
- 18. Aggarwal A, Nossiter J, Cathcart P, et al. Organisation of prostate cancer services in the English National Health Service. Clin Oncol (R Coll Radiol) 2016; 28: 482–489.
- 19. Mitchell JM. Urologists’ use of intensity‐modulated radiation therapy for prostate cancer. N Engl J Med 2013; 369: 1629–1637.
- 20. Nam RK, Cheung P, Herschorn S, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: a population‐based cohort study. Lancet Oncol 2014; 15: 223–231.
- 21. Shakespeare TP, Chin S, Manuel L, et al. Long‐term decision regret after post‐prostatectomy image‐guided intensity‐modulated radiotherapy. J Med Imaging Radiat Oncol 2017; 61: 141–145.
- 22. Smith A, Rincones O, Sidhom M. Robot or radiation? A qualitative study of the decision support needs of men with localised prostate cancer choosing between robotic prostatectomy and radiotherapy treatment. Patient Educ Couns 2019; 102: 1364–1372.
- 23. Wallis CJD, Morton G, Herschorn S, et al. The effect of selection and referral biases for the treatment of localised prostate cancer with surgery or radiation. Br J Cancer 2018; 118: 1399–1405.
- 24. Hall SE, Holman CD, Wisniewski ZS, Semmens J. Prostate cancer: socio‐economic, geographical and private‐health insurance effects on care and survival. BJU Int 2005; 95: 51–58.
- 25. Barbiere JM, Greenberg DC, Wright KA, et al. The association of diagnosis in the private or NHS sector on prostate cancer stage and treatment. J Public Health (Oxf) 2012; 34: 108–114.
- 26. Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer 2005; 103: 1819–1825.
- 27. Mealing NM, Banks E, Jorm LR, et al. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol 2010; 10: 26.
- 28. Evans SM, Nag N, Roder D, et al. Development of an international prostate cancer outcomes registry. BJU Int 2016; 117 (Suppl 4): 60–67.
- 29. Australian Bureau of Statistics. Remoteness structure. https://www.abs.gov.au/websitedbs/d3310114.nsf/home/remoteness+structure (viewed Feb 2021)





Abstract
Objectives: To describe patterns of care in New South Wales for men with prostate cancer, and to ascertain factors associated with receiving different types of treatment.
Design: Individual patient data record linkage study.
Setting, participants: 4003 New South Wales men aged 45 years or more enrolled in the population‐based 45 and Up Study in whom prostate cancer was first diagnosed during 2006–2013.
Main outcome measures: Prostate cancer treatment type received; factors statistically associated with treatment received; proportions of patients who consulted radiation oncologists prior to treatment.
Results: In total, 1619 of 4003 patients underwent radical prostatectomy (40%), 893 external beam radiotherapy (EBRT) (22%), 183 brachytherapy (5%), 87 chemotherapy (2%), 373 androgen deprivation therapy alone (9%), and 848 no active treatment (21%). 205 of 1628 patients who had radical prostatectomies (13%) had radiation oncology consultations prior to surgery. Radical prostatectomy was more likely for patients aged 45–59 years, with regional stage disease, living 100 km or more from the nearest radiotherapy centre, having partners, or having private health insurance, while lower physical functioning, obesity, and living in areas of greater socio‐economic disadvantage reduced the likelihood. EBRT was more likely for patients aged 70–79 years, with non‐localised or unknown stage disease, living less than 100 km from the nearest radiotherapy centre, or not having private health insurance, while the likelihood was lower for patients aged 45–59 years or more than 80 years and for those who had several comorbid conditions.
Conclusions: Men with prostate cancer were twice as likely to have radical prostatectomy as to receive EBRT, and fewer than one in seven had consulted radiation oncologists prior to prostatectomy. The treatment received was influenced by several socio‐demographic factors. Given the treatment‐specific side effects and costs, policies that affect access to different treatments for prostate cancer should be reviewed.