The known: Biosimilar infliximab is safe and clinically effective for a range of disease indications, including inflammatory bowel disease (IBD), but the clinical effectiveness of switching from originator to biosimilar infliximab is less clear.
The new: Switching clinically stable patients with IBD from originator to biosimilar infliximab was safe and clinically non‐inferior to continuing treatment with originator infliximab. The introduction of biosimilar infliximab has also led to cost savings for the Pharmaceutical Benefit Scheme by increasing market competition.
The implications: Our findings should provide assurance to clinicians and patients regarding the safety and clinical effectiveness of biosimilar infliximab, including after non‐medical switching from originator to biosimilar preparations.
Biologic (monoclonal) agents such as infliximab are clinically effective for a range of chronic disease indications, including moderate to severe inflammatory bowel disease (IBD).1,2 However, these medications are expensive, accounting for as much as 64% of IBD‐related health care costs.3 During 2015–16, expenditure for biologic medications in Australia was estimated to total at least $2.3 billion, and cost reduction strategies are needed to ensure the sustainability of biologic therapies for Australian patients.4
One approach to reducing pharmaceutical expenditure is using less expensive biosimilar medicines.4 The action of a biosimilar medication in vitro is very similar to that of the reference product, and there should be no clinically meaningful differences in potency, purity, or safety.5,6,7 Moreover, biosimilar products must meet rigorous standards of safety and efficacy before being approved for clinical use.6,7 Several studies have found that infliximab biosimilars are safe and clinically effective for several indications, including IBD, in infliximab‐naïve patients.5,8,9,10,11 However, it is less clear whether non‐medical switching (ie, switching that is not clinically motivated) of patients with clinically stable IBD from originator infliximab to a biosimilar achieves comparable clinical outcomes.5,12,13
The NOR‐SWITCH randomised controlled trial in Norway evaluated the safety and clinical efficacy of switching patients with inflammatory diseases from originator infliximab to a biosimilar.12 Clinical outcomes were less favourable for patients in the IBD subgroup switched to the infliximab biosimilar (CT‐P13), but the study was not powered to statistically evaluate outcomes for individual disease indications. Subsequent studies that have evaluated the safety and effectiveness of non‐medical switching of patients with IBD have been observational in nature and did not include control (ie, non‐switch) arms.5,14
In this study, we compared clinical and safety outcomes for a large cohort of clinically stable patients with IBD who were switched from originator infliximab to the biosimilar CT‐P13 (Inflectra) with those for patients who continued to receive originator infliximab (Remicade).
Methods
Study design and participants
The Switching Australian patients with Moderate to severe inflammatory bowel diseasE from originator to biosimilar infliximab (SAME) study was a prospective, open label, multi‐centre, parallel cohort, non‐inferiority study of adults (18 years or more old) with moderate to severe IBD who either continued maintenance originator infliximab treatment or were switched to biosimilar CT‐P13 (Inflectra). All patients with a diagnosis of Crohn disease or ulcerative colitis based on standardised clinical, endoscopic, histologic, and radiologic criteria who had been in stable steroid‐free clinical or biochemical remission for at least 12 weeks at enrolment were eligible for participation. Patients receiving limited duration courses of infliximab, with a stoma, or awaiting surgery were excluded.
Our observational study was undertaken from 1 May 2017 to 31 October 2019. From the perspective of clinical practice and pharmacovigilance, it was deemed more appropriate to use a single brand of infliximab at each participating hospital; that is, for the clinicians at each hospital to opt for participation in either the control or switch arms of the study. Patients in four public hospitals were switched from originator infliximab (Remicade) to CT‐P13, while patients in three hospitals (one public, two private) continued to receive originator infliximab (Box 1). Each patient was followed for 48 weeks.
Decision to switch patients to the infliximab biosimilar, CT‐P13
The decision to switch patients with IBD from originator infliximab to CT‐P13 was determined at each participating hospital, independently of this study, according to collaborative discussions between IBD clinicians and the drug and therapeutics committee of the hospital. Reasons for switching included potential cost savings to the hospital and the capacity of a hospital to undertake a managed switch program. Patients identified by their clinicians as clinically suitable for non‐medical switching were advised by mail of the intention to switch them from originator infliximab to CT‐P13; the letter also included information about the clinical similarity, safety, and effectiveness of the biosimilar product. In addition, each patient was phoned by a member of their IBD team to confirm receipt of the letter, to answer their questions, and to confirm whether they had agreed to switching to CT‐P13. Patients could choose to continue treatment with originator infliximab if they did not wish to switch.
Data collection
Data on baseline demographic, disease, and medication characteristics were recorded, with information on the clinical assessment of IBD disease activity, adverse events, and commencement of corticosteroid therapy captured at the time of infusion and at outpatient follow‐up. Blood was collected immediately prior to infliximab administration at baseline and prior to every second infusion during follow‐up for measuring infliximab trough and antibody levels, and C‐reactive protein (CRP) and albumin concentrations.
Study outcomes
The primary outcome was disease worsening and the need for corticosteroid rescue therapy, intensification or discontinuation of maintenance infliximab therapy, or surgery. Secondary outcome measures were time to disease worsening, duration of infliximab treatment persistence, change in infliximab trough levels, and changes in clinical disease indices. Adverse outcomes — including death, infusion reactions, serious infections, and adverse events requiring admission or treatment discontinuation — were also recorded.
Study definitions
Clinical disease activity in patients with Crohn disease was evaluated with the Harvey–Bradshaw Index (HBI)15 and in patients with ulcerative colitis with the partial Mayo Score (pMS).16 Clinical remission was defined as HBI < 5 for patients with Crohn disease, or pMS < 2 for patients with ulcerative colitis. Biochemical remission for patients in either group was defined as CRP < 5 mg/L or faecal calprotectin < 150 μg/mL. Objectively assessed active disease was defined as the presence of biochemical (CRP ≥ 5 mg/L, faecal calprotectin ≥ 150 μg/mL), endoscopic, or radiological signs of inflammation. Disease worsening was defined for patients with Crohn disease as objectively assessed active disease associated with an absolute increase from baseline HBI of at least 4 points and HBI ≥ 7, and for patients with ulcerative colitis as objectively assessed active disease associated with an absolute increase from baseline pMS of at least 3 points and pMS ≥ 5.
Plasma infliximab was assayed with Promonitor‐IFX ELISA kits (Grifols); levels below 3.0 μg/mL were deemed sub‐therapeutic. Anti‐infliximab antibodies — measured if infliximab itself was undetectable — were reported qualitatively (detectable at a level of 10.0 ng/mL). Immunomodulator co‐therapy referred to concomitant use of a thiopurine or methotrexate with maintenance infliximab therapy.
Statistical analysis
Assuming no difference between the control and switch arms in the proportions of people reaching the primary outcome, we estimated that 240 patients (120 per group) were required to ensure, with 90% confidence, that the upper limit of the 95% confidence interval (two‐sided) would exclude a difference in favour of originator infliximab of more than 15 percentage points, the non‐inferiority margin applied in the NOR‐SWITCH study.12
We summarised categorical variables as counts and percentages, and continuous variables as means (with standard deviations [SDs]) or medians (with interquartile ranges [IQRs]). Data for continuous variables were compared in Student unpaired t tests (parametric data) or in Mann–Whitney or Kruskal–Wallis tests (non‐parametric data); categorical variables were compared in Fisher exact tests.
The primary outcome was assessed in an intention to treat analysis; patients who discontinued therapy because of medication intolerance, an adverse event, or non‐compliance were classified as having reached the primary endpoint of disease worsening. The primary outcome was assessed in a linear probability model in a generalised estimating equations framework, adjusted for disease duration, infliximab treatment duration, immunomodulator co‐therapy, baseline dose escalation, and clustering of intervention in centres, and expressed as adjusted hazard ratios (HRs) and risk differences, each with 95% confidence intervals (CIs).
Secondary dichotomous outcomes were analysed by logistic regression, with time to outcome event analysed in Cox regression models. Changes in continuous variables, including infliximab levels and disease clinical activity scores, were analysed in repeated measures models in a generalised estimating equations framework.
All statistical analyses were undertaken in SPSS Statistics 25 (IBM). P < 0.05 was deemed statistically significant.
Ethics approval
Ethics approval for the study was granted by the human research ethics committees of the South Metropolitan Health Service (Perth; reference, RGS0000000543), St Vincent's Hospital (Sydney; reference, LNR/17/SVH/238), Mater Misericordiae (Brisbane; reference, HREC/18/MHS/67), and Eastern Health (Melbourne; reference, LR54‐2018). Patients provided written consent to participating in the study.
Results
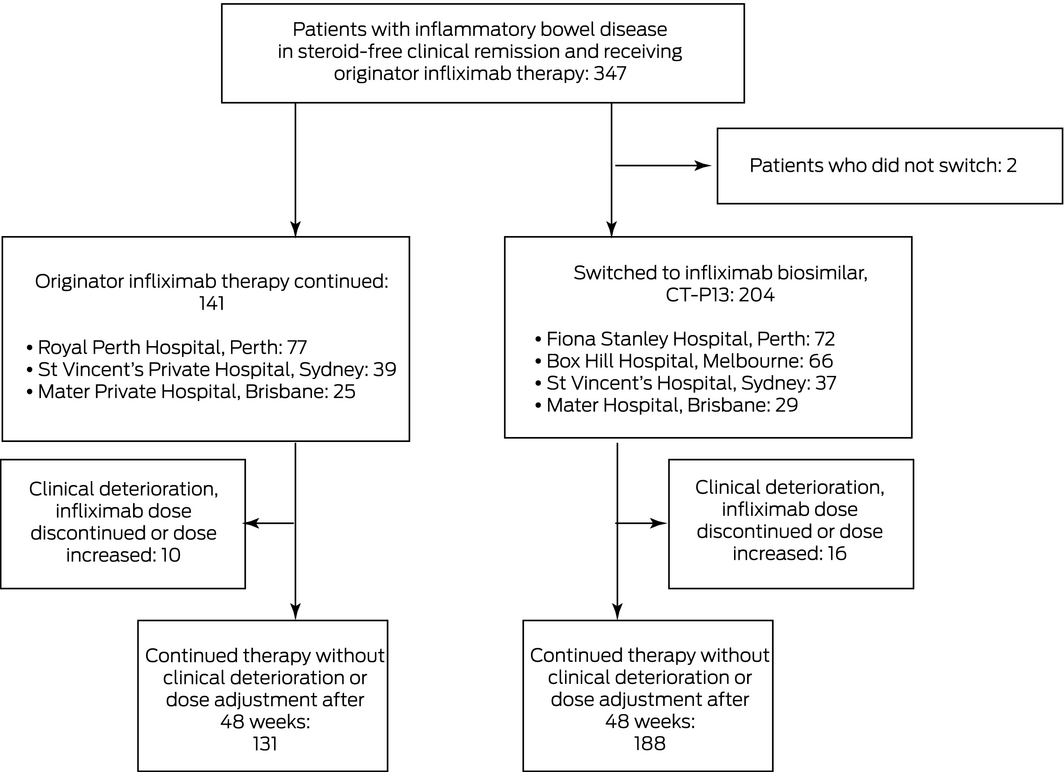
Three‐hundred and forty‐five patients with IBD (including 232 with Crohn disease) were enrolled; 204 (59%) in four hospitals were switched to CT‐P13, 141 (41%) in the three other hospitals continued to receive originator infliximab. Two patients deemed clinically suitable for switching elected to continue originator infliximab, and were excluded from the study (Box 1). The baseline patient, disease, and treatment characteristics of the two groups were similar (Box 2).
Primary outcome
Sixteen patients switched to CT‐P13 (8%) and ten in the control group (7%) experienced clinical deterioration; the adjusted risk difference (control v switch group) was –1.1 percentage points (95% CI, –6.1 to 8.2 percentage points), within the pre‐specified non‐inferiority margin of 15 percentage points (Box 3); the adjusted HR was 1.31 (95% CI, 0.54–3.17) (Box 4, A). Analysed separately, there were no differences between the two arms in estimated risk for the two disease subgroups (Box 3).
Secondary outcomes
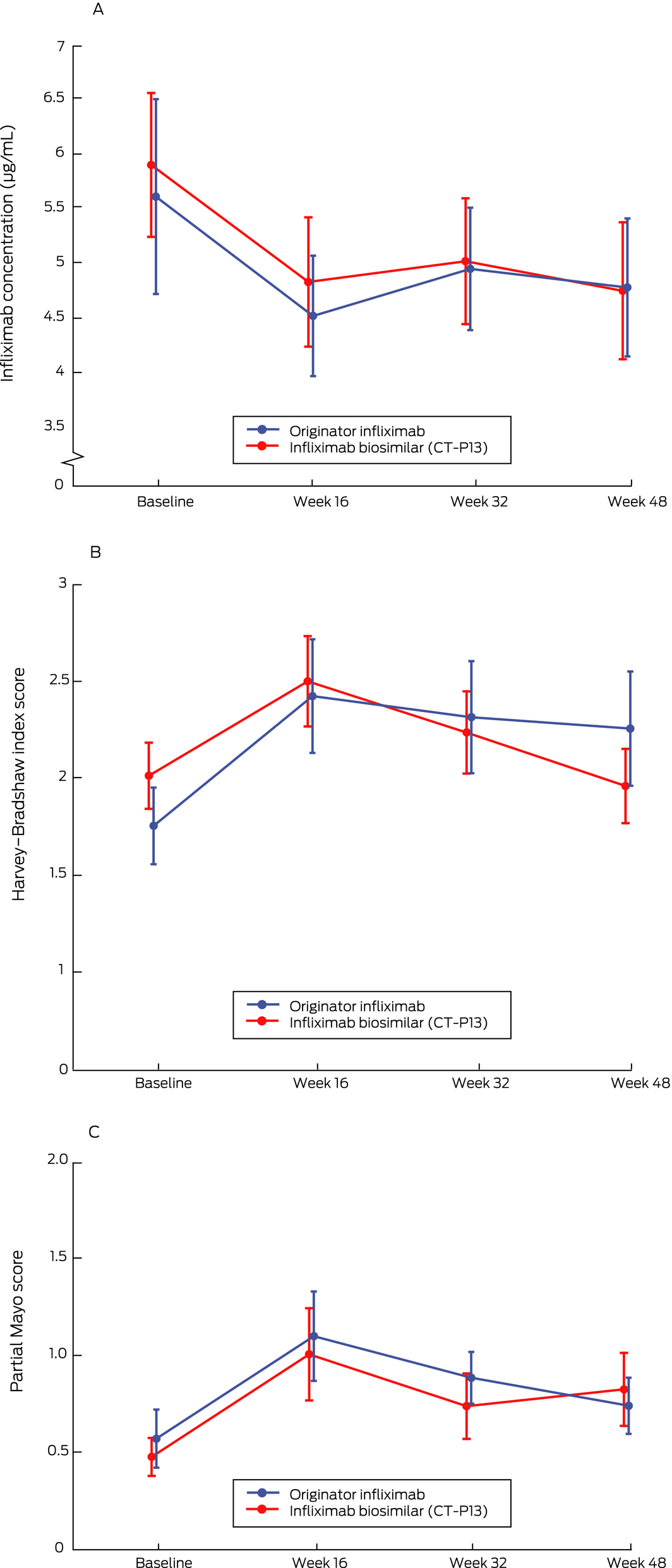
Infliximab treatment persistence over 48 weeks was similar for the two groups: infliximab therapy was intensified or discontinued for 31 patients receiving CT‐P13 (15%) and 20 patients receiving originator infliximab (14%) (adjusted HR, 1.39; 95% CI, 0.71–2.76; Box 4, B). Infliximab trough levels were similar for the two groups at all time points (Box 5, A), as were the proportions of patients with detectable anti‐infliximab antibodies (originator infliximab, 12 [8.5%]; CT‐P13, 15 [7.4%]). Clinical disease activity was also comparable for the two groups throughout the study (Box 5, B,C).
Adverse events
Six patients in each arm of the study experienced adverse events requiring discontinuation of infliximab therapy. Serious adverse events leading to infliximab discontinuation in the switch group were exacerbation of pre‐existing joint pain, drug‐related anaphylaxis, de novo vasculitis, and fatal metastatic lung cancer; in the control group, reasons for discontinuing infliximab were acute joint pain, recurrent furunculitis, severe infection requiring hospitalisation, and acute myeloid leukaemia. There were two infusion‐related reactions in the switch group, three in the control group (Box 6).
Discussion
The results of the SAME study indicate that non‐medical switching of Australian patients with IBD from originator to biosimilar infliximab is a safe and clinically non‐inferior alternative to continuing treatment with originator infliximab.
Our findings are consistent with overseas reports that switching from originator to biosimilar infliximab is safe and not accompanied by increased numbers of adverse drug events or infusion reactions.17,18,19,20 Severe adverse events requiring discontinuation of infliximab therapy were rare in both groups in our study, and could not be linked with switching from originator to biosimilar infliximab. Moreover, infliximab trough and antibody levels were consistently similar for the two groups across the study, providing assurance that the pharmacokinetics of originator and biosimilar infliximab are similar. The large study cohort also facilitated separate comparison of outcomes for people with Crohn disease or ulcerative colitis. Outcomes were also similar for the control and switch groups (Box 3), although our study was not adequately powered to draw definitive statistical conclusions about IBD subgroups.
The SAME study is one of the largest controlled observational studies to evaluate the safety and clinical effects of non‐medical switching of patients with IBD. Our study also assures Australian health professionals and patients that switching from originator to biosimilar infliximab is safe and non‐inferior to continuing treatment with originator infliximab. Moreover, given the relatively low rates of clinical disease worsening and of adverse events in people switched to CT‐P13, a marked nocebo effect, as suggested by the authors of another investigation of infliximab switching,21 was not apparent. Further, infliximab drug and antibody testing at multiple time points over 48 weeks did not detect any changes associated with switching to biosimilar infliximab.
Further, our findings have significant health economic implications. At study commencement (May 2017), the Pharmaceutical Benefits Scheme (PBS) reimbursed $574.85 per 100 mg infliximab vial; at study conclusion (October 2019), the rate was $320.71 per vial.22 On the basis of six PBS‐funded 8‐weekly infliximab infusions over 48 weeks per patient, the estimated annualised cost to the PBS for the entire study group (total of 1547 vials) in May 2017 was $5.34 million, and $2.98 million in October 2019. That is, the estimated annual cost to the PBS was $2.36 million lower in October 2019 (44%), or $6837 less per patient‐year of infliximab therapy. This cost saving, independent of whether originator or biosimilar infliximab or CT‐P13 is used for therapy, was the result of increased market competition following the introduction of biosimilar infliximab in Australia for the treatment of IBD.
Limitations
First, all patients who switched from originator to biosimilar infliximab were treated in public tertiary IBD centres, while 64 of 141 patients in the control group (45%) were infused in private hospitals. Although we adjusted our analyses for patient, disease, and treatment characteristics, as well as for clustering by site, unmeasured demographic differences between the control and switch groups were possible. Second, we evaluated the effect of switching in patients in steroid‐free clinical remission, and our findings may not be generalisable to patients with clinically active disease. Third, while the pre‐defined non‐inferiority margin of 15 percentage points was taken from the NOR‐SWITCH study,12 it may be too wide for establishing genuine non‐inferiority. Fourth, although rates of adverse events leading to discontinuation of infliximab treatment were similar to those in the NOR‐SWITCH study in both the switch and control groups (3–4%), the rates of non‐serious adverse events in our study may have been influenced by differences between hospitals in their reporting and documentation. Finally, we did not evaluate the safety of multiple switches between originator and biosimilar infliximab.
Conclusions
The results of the SAME study indicate that it is safe to switch clinically stable patients with moderate to severe IBD from originator to biosimilar infliximab. The introduction of the infliximab biosimilar CT‐P13 (Inflectra) has also led to a considerable reduction in the PBS‐listed price for infliximab, resulting in millions of dollars in estimated cost savings for the PBS. It is therefore anticipated that the SAME study will reassure both health professionals and patients that biosimilar infliximab is safe, clinically effective, and economical.
Box 1 – Flow of patients through the study: clinically stable patients with inflammatory bowel disease treated with originator infliximab (Remicade) or switched to an infliximab biosimilar (CT‐P13, Inflectra) for 48 weeks
Box 2 – Baseline patient and disease characteristics
|
Characteristic |
Originator infliximab (control) |
Infliximab biosimilar (switch) |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
141 |
204 |
|||||||||||||
|
Patients |
|
|
|||||||||||||
|
Sex (men) |
84 (60%) |
109 (53%) |
|||||||||||||
|
Age (years), median (IQR) |
35.6 (18.2–72.8) |
36.4 (16.8–86.5) |
|||||||||||||
|
Disease duration at enrolment (years), median (IQR) |
7.0 (0.5–40) |
9.1 (0.3–50) |
|||||||||||||
|
Inflammatory bowel disease |
|
|
|||||||||||||
|
Crohn disease |
91 (65%) |
141 (69%) |
|||||||||||||
|
Ulcerative colitis |
50 (35%) |
63 (31%) |
|||||||||||||
|
Treatment |
|
|
|||||||||||||
|
Infliximab duration at enrolment (years), median (IQR) |
3.0 (0.5–40) |
3.3 (0.3–50) |
|||||||||||||
|
Immunomodulator co‐therapy* |
58 (50%) |
99 (57%) |
|||||||||||||
|
Baseline assessments |
|
|
|||||||||||||
|
Harvey–Bradshaw index, median (IQR) |
2 (0–5) |
1 (0–6) |
|||||||||||||
|
Partial Mayo score, median (IQR) |
0 (0–2) |
0 (0–2) |
|||||||||||||
|
C‐reactive protein (mg/L), median (IQR) |
1.5 (0.3–60) |
2.9 (0.3–42) |
|||||||||||||
|
Infliximab level (μg/mL), median (IQR) |
4.9 (0.5–16) |
5.5 (0.1–18) |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Concurrent azathioprine, 6‐mercaptapurine, or methotrexate. |
|||||||||||||||
Box 3 – Disease worsening (requiring discontinuation or intensification of infliximab therapy) in patients with inflammatory bowel disease: estimated risk differences
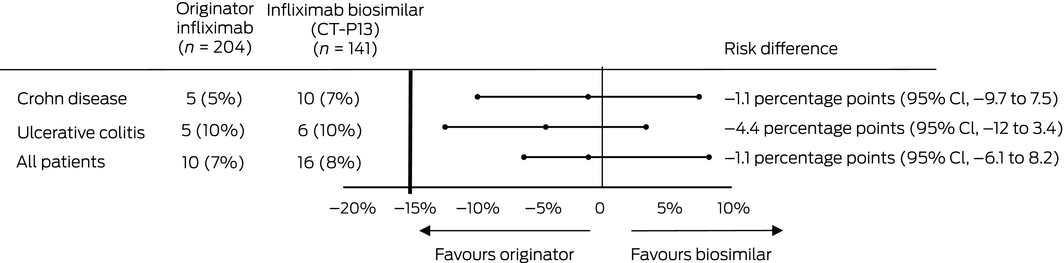
CI = confidence interval.
Box 4 – Persistence of infliximab therapy without dose intensification or change from infliximab to another biologic. A. Censored by disease worsening (primary outcome);* B. Censored by infliximab intensification or discontinuation (any reason)
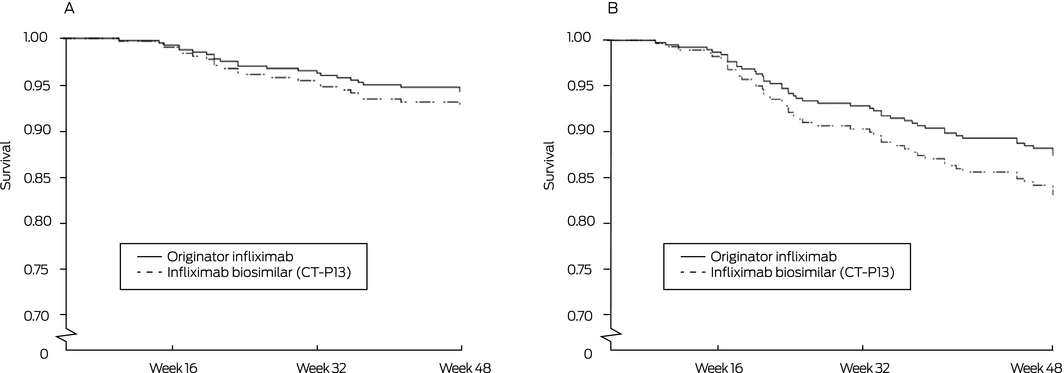
* Objectively assessed active disease associated with a Harvey–Bradshaw Index score of at least 7, and at least 4 points higher than at baseline (Crohn disease) or with a partial Mayo score of at least 5 points, and at least 3 points higher than at baseline (ulcerative colitis).
Received 31 March 2020, accepted 27 July 2020
- Craig Haifer1,2
- Ashish Srinivasan3,4
- Yoon‐Kyo An5,6
- Sherman Picardo7
- Daniel Langenberg3,4
- Shankar Menon7
- Jakob Begun5,8
- Simon Ghaly1,9
- Lena Thin10,11
- 1 St Vincent's Hospital Sydney, Sydney, NSW
- 2 The University of Sydney, Sydney, NSW
- 3 Eastern Health, Melbourne, VIC
- 4 Monash University Eastern Health Clinical School, Melbourne, VIC
- 5 Mater Hospital Brisbane, Brisbane, QLD
- 6 The University of Queensland, Brisbane, QLD
- 7 Royal Perth Hospital, Perth, WA
- 8 Mater Research Institute, University of Queensland, Brisbane, QLD
- 9 St Vincent's Clinical School, Sydney, NSW
- 10 Fiona Stanley Hospital, Perth, WA
- 11 The University of Western Australia, Perth, WA
Pfizer provided an unrestricted grant to fund testing of infliximab drug and antibody levels. Pfizer had no role in study design, analysis, or reporting of this data.
Craig Haifer has received speaker fees and educational support from Janssen, Pfizer, Takeda, Ferring and AbbVie. Yoon‐Kyo An has received speaker fees and educational support from Janssen, Pfizer, Takeda and AbbVie. Sherman Picardo has received speaker fees from AbbVie, Janssen, Pfizer and Takeda. Daniel van Langenberg has received educational grants and research support from Pfizer, Takeda, Ferring and Shire, and has received consultancy and speaker fees from Pfizer, Janssen, AbbVie, Ferring, Vifor Pharma and Emerge Health. Lena Thin has received speaker fees, advisory board fees and education grant support from Pfizer, AbbVie, Takeda and BMS.
- 1. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549.
- 2. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476.
- 3. van der Valk ME, Mangen MJJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFα therapy: results from the COIN study. Gut 2014; 63: 72–79.
- 4. Gleeson D, Townsend B, Lopert R, et al. Financial costs associated with monopolies on biologic medicines in Australia. Aust Health Rev 2019; 43: 36–42.
- 5. Moayyedi P, Benchimol EI, Armstrong D, et al. Joint Canadian Association of Gastroenterology and Crohn's Colitis Canada position statement on biosimilars for the treatment of inflammatory bowel disease. J Can Assoc Gastroenterol 2020; 3: e1–e9.
- 6. Weise M, Bielsky MC, de Smet K, et al. Biosimilars: what clinicians should know. Blood 2012; 120: 5111–5117.
- 7. Lemery SJ, Ricci MS, Keegan P, et al. FDA's approach to regulating biosimilars. Clin Cancer Res 2017; 23: 1882–1885.
- 8. Park W, Hrycaj P, Jeka S, et al. A randomised, double‐blind, multicentre, parallel‐group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT‐P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013; 72: 1605–1612.
- 9. Meyer A, Rudant J, Drouin J, et al. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: a French equivalence study. Ann Intern Med 2019; 170: 99–107.
- 10. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double‐blind, parallel‐group study to demonstrate equivalence in efficacy and safety of CT‐P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013; 72: 1613–1620.
- 11. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT‐P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double‐blind, phase 3 non‐inferiority study. Lancet 2019; 393: 1699–1707.
- 12. Jørgensen KK, Olsen IC, Goll GL, et al; NOR‐SWITCH study group. Switching from originator infliximab to biosimilar CT‐P13 compared with maintained treatment with originator infliximab (NOR‐SWITCH): a 52‐week, randomised, double‐blind, non‐inferiority trial. Lancet 2017; 389: 2304–2316.
- 13. Nguyen E, Weeda ER, Sobieraj DM, et al. Impact of non‐medical switching on clinical and economic outcomes, resource utilization and medication‐taking behavior: a systematic literature review. Curr Med Res Opin 2016; 32: 1281–1290.
- 14. Feagan BG, Lam G, Ma C, Lichtenstein GR. Systematic review: efficacy and safety of switching patients between reference and biosimilar infliximab. Aliment Pharmacol Ther 2019; 49: 31–40.
- 15. Harvey R, Bradshaw MJ. Measuring Crohn's disease activity. Lancet 1980; 315: 1134–1135.
- 16. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 1987; 317: 1625–1629.
- 17. Razanskaite V, Bettey M, Downey L, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis 2017; 11: 690–696.
- 18. Bergqvist V, Kadivar M, Molin D, et al. Switching from originator infliximab to the biosimilar CT‐P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol 2018; 11: 1–13.
- 19. Plevris N, Jones GR, Jenkinson PW, et al. Implementation of CT‐P13 via a managed switch programme in Crohn's disease: 12‐month real‐world outcomes. Dig Dis Sci 2019; 64: 1660–1667.
- 20. Chaparro M, Garre A, Guerra Veloz MF, et al. Effectiveness and safety of the switch from Remicade® to CT‐P13 in patients with inflammatory bowel disease. J Crohns Colitis 2019; 13: 1380–1386.
- 21. Tweehuysen L, van den Bemt BJ, van Ingen IL, et al. Subjective complaints as the main reason for biosimilar discontinuation after open‐label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol 2018; 70: 60–68.
- 22. Pharmaceutical Benefits Scheme. Infliximab. https://www.pbs.gov.au/medicine/item/4284L-5753T-5754W-5755X-5756Y-5757B-5758C-6397Q-6448J-6496X-9612X-9613Y-9617E-9654D-9674E (viewed Mar 2020).





Abstract
Objective: To examine whether non‐medical switching of patients with inflammatory bowel disease (IBD) from originator infliximab to a biosimilar (CT‐P13, Inflectra) is safe and clinically non‐inferior to continued treatment with originator infliximab.
Design: Prospective, open label, multicentre, parallel cohort, non‐inferiority study in seven Australian hospitals over 48 weeks, May 2017 – October 2019.
Participants: Adults (18 years or older) with IBD receiving maintenance originator infliximab (Remicade) who had been in steroid‐free clinical remission for at least 12 weeks.
Intervention: Managed program for switching patients in four hospitals from originator to biosimilar infliximab (CT‐P13); patients in three other hospitals continued to receive originator infliximab (control).
Main outcome measures: Clinical disease worsening requiring infliximab dose escalation or change in therapy.
Results: The switch group included 204 patients, the control group 141 patients with IBD. Ten patients in the control group (7%) and 16 patients switched to CT‐P13 (8%) experienced clinical deterioration; the adjusted risk difference (control v switch group) was –1.1 percentage points (95% CI, –6.1 to 8.2 percentage points), within our pre‐specified non‐inferiority margin of 15 percentage points. Serious adverse events leading to infliximab discontinuation were infrequent in both the switch (six, 3%) and control (six, 4%) groups.
Conclusion: Switching patients with IBD from originator to biosimilar infliximab is safe and non‐inferior to continuing treatment with originator infliximab. Moreover, the introduction of biosimilar infliximab, by increasing market competition, has resulted in substantial cost savings for the Pharmaceutical Benefits Scheme.