The known: Corticosteroid therapy did not improve clinical outcomes for patients with SARS or MERS, and the WHO recommends against treating patients with COVID‐19 with corticosteroids, although medical societies in China recommend their prudent use.
The new: Corticosteroid therapy is widely employed to treat patients with COVID‐19 in Wuhu, China, but we found no evidence of clinical benefit for those without acute respiratory distress syndrome. Virus clearance may be slower in people with chronic HBV infections.
The implications: The value of corticosteroid therapy for patients with COVID‐19 should be reconsidered, and the effect of other infections on viral clearance investigated.
In December 2019, a cluster of patients with primary pneumonia caused by a novel coronavirus was reported in Wuhan, China. The virus subsequently spread rapidly throughout the country and around the world.1,2,3 The novel coronavirus was later identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2); it exhibits 79.5% identity with the SARS‐CoV that caused the 2003 SARS epidemic.4 The disease caused by the new virus, coronavirus disease 2019 (COVID‐19), has clinical manifestations ranging from asymptomatic, mild pneumonia to serious acute respiratory distress syndrome (ARDS), septic shock, and multiple organ dysfunction syndrome (MODS).5,6,7 By 23 March 2020, 81 601 cases of COVID‐19 had been reported in mainland China, and the general mortality rate was 4.0%.8 The mortality rate among critically ill patients (61.5%) is similar to that previously reported for the Middle East respiratory syndrome (MERS), also caused by a coronavirus.5,9 The mainstay of management of patients with COVID‐19 is supportive therapy, including fluid management, oxygen therapy, and mechanical ventilation.10
Cytokine storm and inflammation induced by the uncontrolled immunologic response to the virus underlies the fatal pneumonia that can follow infection with human coronaviruses.11,12 Inhibition of inflammation improves outcomes in animals infected with SARS and MERS viruses.13,14 Corticosteroids are typically used to treat severe acute respiratory infections of viral aetiology because of their anti‐inflammatory effect.15 However, it was reported that corticosteroids did not improve outcomes during the SARS and MERS outbreaks, but delayed viral clearance and increased rates of secondary infections.9,16,17 Most patients in the relevant studies were critically ill with ARDS, and may have passed the point where adverse outcomes could be modified by steroid therapy.
The World Health Organization has recommended against routinely administering systemic corticosteroids to patients with COVID‐19.18 Nevertheless, a consensus statement by the Chinese Thoracic Society recommends using corticosteroids, albeit prudently,19 ideally in the context of a randomised controlled trial. To undertake such a trial during an ongoing epidemic is challenging. To facilitate study design, we report the impact of corticosteroid therapy in patients with COVID‐19 according to accumulated observational clinical data.
Methods
Study design and participants
Our observational study was undertaken in the Second People's Hospital of Wuhu and Yijishan Hospital, the two designated hospitals for patients with COVID‐19 in Wuhu, Anhui province, about 500 km from Wuhan. The two tertiary teaching hospitals (4000 beds total) are the only units authorised to admit infectious patients with COVID‐19 in Wuhu. We reviewed the records of patients admitted to the hospitals with the diagnosis of COVID‐19 during 24 January – 24 February 2020. Laboratory confirmation of SARS‐CoV‐2 infection was made by the local Centre for Disease Control, using reverse transcription polymerase chain reaction (RT‐PCR) as reported previously.2
Data collection
Data extracted from patient records included age, sex, comorbid conditions, symptoms at onset, time from onset of symptoms to hospital admission, vital signs at admission, laboratory test results during hospitalisation, computed tomography (CT) imaging findings, virus clearance time, treatments (duration and dosage), other infections, and clinical outcomes. The record included the field “exposure to Wuhan”, defined as travel to Wuhan City, Hubei, or close contact with someone recently arrived from Wuhan City.
Outcomes and definitions
The primary outcome was time to virus clearance. Secondary outcomes included duration of clinical recovery and length of hospital stay.
Viral clearance was confirmed by serial RT‐PCR of samples from throat swabs; clearance was defined as having at least two consecutive negative results for swabs collected 24 hours apart. The virus clearance time was calculated from the onset of symptoms to the date of the first negative RT‐PCR test. Clinical recovery was determined by the attending physicians by integrating information about clinical manifestations, laboratory test results, and radiological results. Patients were characterised as having received corticosteroids if they received at least one dose during their hospital stay.
Statistical analysis
Continuous variables were summarised as medians with interquartile ranges (IQRs); categorical variables were summarised as frequencies and percentages. The statistical significance of differences between patients receiving and not receiving corticosteroids were assessed in Fisher exact tests or Wilcoxon signed‐rank tests (categorical variables) and Mann–Whitney U tests (continuous variables). The association between corticosteroid therapy and time to outcomes was assessed in a survival analysis. Univariable hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. P < 0.05 was deemed statistically significant. All analyses were performed in R 3.6.2.
Ethics approval
The study was approved by the ethics committee of the Second People's Hospital of Wuhu (application reference, 202001).
Results
Patient characteristics and clinical presentation
Thirty‐one patients were included in the study. Their median age was 39 years (IQR, 32–54 years); 20 were men (64%). Twenty‐one patients (68%) had returned from Wuhan, the centre of the COVID‐19 epidemic in China, where they were presumed to have contracted the virus.
Comorbid conditions were not frequent: seven patients had hypertension, two had chronic hepatitis B virus (HBV) infections (virus loads, 2950 and 3040 copies/mL; both patients were receiving entecavir), one had diabetes, and one had coronary heart disease. Two patients had histories of smoking. No patients reported chronic respiratory diseases, cancer, or other chronic diseases.
On admission, patients presented with signs and symptoms of systemic infection; most reported fever, cough, and myalgia or fatigue (Box 1). Five patients reported diarrhoea. All patients in the study were classified as having mild disease: only four reported dyspnoea, and none developed ARDS. The median time from illness onset to admission was four days (IQR, 2–6 days).
Laboratory and radiological findings
Five patients had leukopenia (counts below 4 × 109/L), nine had lymphopenia (< 1.0 × 109/L), and two had platelet counts below 100 × 109/L. C‐reactive protein (CRP) concentration exceeded 10 mg/L in 20 patients (reference range, < 3.0 mg/L), while procalcitonin levels were within the normal range (0.1–0.5 ng/mL) in 29 patients. In six patients, alanine aminotransferase levels were elevated (≥ 40 U/L), as was lactate dehydrogenase in five patients (≥ 225 U/L). The creatine kinase level was elevated in two patients (≥ 175 U/L); while cardiac troponin levels were normal in all patients (reference range < 0.5 ng/mL) (Supporting Information).
For 29 of the 31 patients, pneumonia was evident on chest CT imaging, including 20 with bilateral involvement (Supporting Information); for two patients, no abnormalities were evident on chest CT at any point. The typical radiological changes were bilateral or bilobular ground glass opacity that usually progressed within one week (Box 2).
Treatments
All patients received lopinavir/ritonavir (protease inhibitors) and interferon alfa (an antiviral agent) by inhalation; this combination was previously used to treat patients with MERS.20 Five patients also received umifenovir, which blocks viral endocytosis and replication of a large panel of viruses; it is licensed in Russia and China for treating and preventing influenza.21 Moxifloxacin monotherapy was used as prophylactic antimicrobial treatment in 14 patients for a median 6.5 days (IQR, 3.5–7.0 days).
Corticosteroid treatment
Corticosteroid (40 mg methylprednisolone once or twice per day) was administered to 11 patients within 24 hours of admission for a median 5 days (IQR, 4.5–5.0 days) (Box 3). Patients who received corticosteroid treatment had a higher maximum temperature on admission than patients who did not receive corticosteroid treatment (38.8°C [IQR, 38.2–39.0°C] v 37.8°C [IQR, 37.0–38.1°C]; P = 0.002), and more frequently reported clinical symptoms on admission, including myalgia or fatigue (11 of 11 [100%] v 8 of 20 [40%]; P = 0.004) and cough (10 of 11 [91%] v 8 of 20 [40%]; P = 0.018) (Box 1). Moreover, patients receiving corticosteroid treatment had higher median CRP levels (84.0 mg/L [IQR, 18.6–150 mg/L v 18.7 mg/L [IQR, 4.77–29.6 mg/L; P = 0.026) and lower median lymphocyte count (0.99 × 109/L [IQR, 0.88–1.29 × 109/L) v 1.54 × 109/L [IQR, 1.25–1.77 × 109/L]; P = 0.012) than patients who did not receive corticosteroid. bilateral involvement was more frequently evident on chest CT of these patients (11 of 11 [100%] v 9 of 20 [45%]; P = 0.009) (Supporting Information).
Outcomes
By 29 February 2020, 26 of the 31 patients (84%) had recovered from COVID‐19 and were discharged well, and five were still hospitalised. The median time to virus clearance was 14 days (IQR, 12–16 days; range, 7–26 days). Median duration of symptoms was 7 days (IQR, 5–10 days); median hospital length of stay was 18.5 days (IQR, 16–21 days). There were no statistically significant differences in virologic or clinical outcomes between patients who received and those who did not receive corticosteroid treatment (Box 3). Interestingly, in unplanned analyses we found an association between existing HBV infection and prolonged virus clearance (mean difference, 10.6 days; 95% CI, 6.2–15.1 days; P < 0.001).
In the survival analysis, times to virus clearance (HR, 1.26; 95% CI, 0.58–2.74; P = 0.55), to discharge (HR, 0.77; 95% CI, 0.33–1.78; P = 0.54), and to clinical resolution of symptoms (HR, 0.86; 95% CI, 0.40–1.83; P = 0.70) were not influenced by corticosteroid treatment.
Discussion
Patients with COVID‐19 are often treated with a corticosteroid despite the lack of clinical evidence for the efficacy of such treatment. In our study, 11 of 31 patients received corticosteroid treatment, a proportion similar to that reported by an earlier study.6 Systematic use of corticosteroid treatment is even higher in critically ill patients with COVID‐19, as many as 70% of these patients receiving it.5 In our study, patients treated with a corticosteroid had more clinical symptoms, a higher inflammation index, and more abnormalities on chest CT, indicating that its use was related to the severity of symptoms on presentation. This practice is consistent with that reported by another study, in which 33% of patients with symptoms of COVID‐19 for more than 10 days had received a corticosteroid, but only 17% of those who had had symptoms for fewer than 10 days.22
Our findings indicate that corticosteroid treatment did not influence virus clearance time, hospital length of stay, or duration of symptoms in patients with mild COVID‐19. Pathology findings indicate that ARDS plays a crucial role in COVID‐19 cases with fatal outcomes,23 and early administration of corticosteroids might reduce the risk of ARDS in virus infections.24 However, we could not assess the efficacy of early corticosteroid therapy for preventing ARDS, as none of our patients developed it.
In a univariate analysis, we found an association between having a chronic HBV infection and longer time to virus clearance. The small number of patients infection (two) does not permit any firm conclusions, but a similar association has previously been reported for SARS‐CoV‐infected patients.25 T cell dysfunction in responses to other viruses in patients with chronic HBV infections26 might explain the longer virus clearance time in patients with COVID‐19, and this association merits further study.
Evidence for the efficacy of drugs used for treating patients with COVID‐19 is derived from research in patients with SARS, MERS, or influenza, and from laboratory studies.27 The small sample in our observational study precludes us from drawing firm conclusions about these treatments. A recent retrospective observational study that compared treating patients with COVID‐19 with umifenovir and interferon alfa inhalation, lopinavir/ritonavir and interferon alfa inhalation, or interferon alfa inhalation alone did not find any statistically significant differences in virus clearance time or duration of symptoms.28 Evidence from formal randomised controlled trials is required to provide definitive guidance on the use of these agents.
Limitations
Our sample size was small, and the patients included in our study were relatively young (median age, 39 years) and had mild disease, limiting the generalisability of our findings to patients without ARDS, the main threat and real challenge in clinical practice. Our small sample size and the observational nature of our study also means that many confounders may have influenced our results. Despite these limitations, our study provides early findings in the context of a rapidly evolving situation.
Conclusion
In our small observational study of patients with mild COVID‐19, overall outcomes were good and not significantly associated with a specific therapy. An existing HBV infection may delay virus clearance, and this association merits further investigation. We will continue to assimilate data and recommend a formal review as more observational and randomised controlled datasets become available.
Box 1 – Baseline characteristics of 31 patients with coronavirus disease 2019 (COVID‐19)
|
Characteristics |
All patients |
Therapy |
P |
||||||||||||
|
Non‐corticosteroid |
Corticosteroid |
||||||||||||||
|
|
|||||||||||||||
|
Number of patients |
31 |
20 |
11 |
|
|||||||||||
|
Age (years), median (IQR) |
39 (32–54) |
37 (27–52) |
53 (36–57) |
0.18 |
|||||||||||
|
Sex |
|
|
|
0.75 |
|||||||||||
|
Men |
20 (64%) |
12 (60%) |
8 (73%) |
|
|||||||||||
|
Women |
11 (36%) |
8 (40%) |
3 (27%) |
|
|||||||||||
|
Comorbid conditions |
|
|
|
|
|||||||||||
|
Hypertension |
7 (23%) |
5 (25%) |
2 (18%) |
1.0 |
|||||||||||
|
Diabetes |
1 (3%) |
0 |
1 (9%) |
0.76 |
|||||||||||
|
Coronary heart disease |
1 (3%) |
1 (5%) |
0 |
1.0 |
|||||||||||
|
Chronic hepatitis B virus infection |
2 (6%) |
2 (10%) |
0 |
0.75 |
|||||||||||
|
Current smoker |
2 (6%) |
1 (5%) |
1 (9%) |
1.0 |
|||||||||||
|
Exposure to Wuhan |
21 (68%) |
11 (55%) |
10 (91%) |
0.10 |
|||||||||||
|
Signs and symptoms |
|
|
|
|
|||||||||||
|
Fever |
25 (81%) |
14 (70%) |
11 (100%) |
0.12 |
|||||||||||
|
Highest temperature (˚C) |
38.0 (37.6–38.8) |
37.8 (37.0–38.1) |
38.8 (38.2–39.0) |
0.002 |
|||||||||||
|
Cough |
19 (61%) |
8 (40%) |
11 (100%) |
0.004 |
|||||||||||
|
Myalgia or fatigue |
18 (58%) |
8 (40%) |
10 (91%) |
0.018 |
|||||||||||
|
Headache |
4 (13%) |
1 (5%) |
3 (27%) |
0.23 |
|||||||||||
|
Diarrhea |
5 (16%) |
5 (25%) |
0 |
0.19 |
|||||||||||
|
Dyspnoea |
4 (13%) |
1 (5%) |
3 (27%) |
0.23 |
|||||||||||
|
Respiratory rate (per minute) |
20 (18.5–20) |
19 (18–20) |
20 (19–21) |
0.18 |
|||||||||||
|
Heart rate (per minute) |
84 (75–95) |
82.5 (73.5–94) |
84 (79–98.5) |
0.32 |
|||||||||||
|
Systolic pressure (mmHg) |
127 (122–137) |
125.5 (117–137) |
128 (125–135) |
0.28 |
|||||||||||
|
Diastolic pressure (mmHg) |
73 (69.5–78.5) |
72.5 (70–86) |
73 (68.5–75) |
0.37 |
|||||||||||
|
Peripheral oxygen saturation (%) |
98 (96–98.5) |
98 (97–99) |
97 (95–98) |
0.11 |
|||||||||||
|
Time from illness onset to hospital admission (days), median (IQR) |
4 (2–6) |
4 (2–5.25) |
4 (2–7.5) |
0.53 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. |
|||||||||||||||
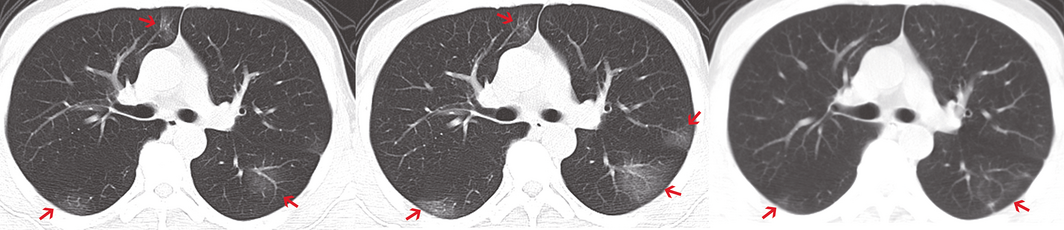
Box 2 – Chest computed tomography (CT): typical findings, including bilateral involvement and ground glass opacities (arrows) that resolved within one week
Box 3 – Treatments and clinical outcomes for 31 patients with coronavirus disease 2019 (COVID‐19)
|
Treatments and outcomes |
All patients |
Therapy |
P |
||||||||||||
|
Non‐corticosteroid |
Corticosteroid |
||||||||||||||
|
|
|||||||||||||||
|
Number of patients |
31 |
20 |
11 |
|
|||||||||||
|
Treatments |
|
|
|
|
|||||||||||
|
Antibiotics (all) |
15 (48%) |
7 (35%) |
8 (73%) |
0.10 |
|||||||||||
|
Moxifloxacin |
14 (45%) |
6 (30%) |
8 (73%) |
0.06 |
|||||||||||
|
Duration of moxifloxacin (days), median (IQR) |
6.5 (3.5–7.0) |
7 (5.5–7) |
7 (6–8.75) |
0.31 |
|||||||||||
|
Lopinavir/ritonavir and interferon alfa |
26 (84%) |
16 (80%) |
10 (91%) |
0.78 |
|||||||||||
|
Umifenovir and lopinavir/ritonavir and interferon alfa |
5 (16%) |
4 (20%) |
1 (9%) |
|
|||||||||||
|
Duration of interferon alfa (days), median (IQR) |
15 (10–17) |
14.5 (10.5–17) |
16 (10.5–17.5) |
0.79 |
|||||||||||
|
Duration of antiviral drug (days), median (IQR) |
10 (8–11.5) |
10 (7.75–13) |
9 (8–10) |
0.48 |
|||||||||||
|
Outcomes |
|
|
|
|
|||||||||||
|
Recovered |
26 (84%) |
15 (75%) |
11 (100%) |
0.19 |
|||||||||||
|
Died |
0 |
0 |
0 |
NA |
|||||||||||
|
Virus clearance time (days), median (IQR) |
14 (11.5–16) |
14 (11–17) |
15 (14–16) |
0.87 |
|||||||||||
|
Duration of symptoms (days), median (IQR) |
7 (5–10.5) |
6.5 (4–9.25) |
8 (5–12) |
0.47 |
|||||||||||
|
Length of hospital stay (days), median (IQR) |
18.5 (16–21) |
17 (15.5–19.5) |
20 (18–21) |
0.14 |
|||||||||||
|
Kidney injury |
0 |
0 |
0 |
— |
|||||||||||
|
Liver injury |
12 (39%) |
7 (35%) |
5 (46%) |
0.85 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; NA = not applicable. |
|||||||||||||||
Received 29 February 2020, accepted 13 March 2020
- Lei Zha1
- Shirong Li2
- Lingling Pan3
- Boris Tefsen1
- Yeshan Li2
- Neil French4
- Liyun Chen5
- Gang Yang2
- Elmer V Villanueva1
- 1 Xi'an Jiaotong–Liverpool University, Suzhou, Jiangsu, China
- 2 The Second People's Hospital of Wuhu, Wuhu, Anhui, China
- 3 Yijishan Hospital of Wannan Medical College, Wuhu, Anhui, China
- 4 Institute of Infection and Global Health, University of Liverpool, Liverpool, United Kingdom
- 5 The Third People's Hospital of Wuhu, Wuhu, Anhui, China
We thank everyone fighting the epidemic of COVID‐19 around the world.
No relevant disclosures.
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506.
- 3. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–936.
- 4. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273.
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; https://doi.org/10.1016/s2213-2600(20)30079-5 [Epub ahead of print].
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069.
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513.
- 8. World Health Organization. Coronavirus disease 2019 (COVID‐19). Situation report 63. 23 Mar 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200323-sitrep-63-covid-19.pdf?sfvrsn=d97cb6dd_2 (viewed 24 Mar 2020).
- 9. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197: 757–767.
- 10. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res 2020; 7: 4.
- 11. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39: 529–539.
- 12. Zhou J, Chu H, Li C, et al. Active MERS‐CoV replication and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis 2014; 209: 1331–1342.
- 13. Chan JF, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015; 212: 1904–1913.
- 14. DeDiego ML, Nieto‐Torres JL, Regla‐Nava JA, et al. Inhibition of NF‐κB‐mediated inflammation in severe acute respiratory syndrome coronavirus‐infected mice increases survival. J Virol 2014; 88: 913–924.
- 15. Sibila O, Agusti C, Torres A. Corticosteroids in severe pneumonia. Eur Respir J 2008; 32: 259–264.
- 16. Hui DS. Systemic corticosteroid therapy may delay viral clearance in patients with Middle East respiratory syndrome coronavirus infection. Am J Respir Crit Care Med 2018; 197: 700–701.
- 17. Auyeung TW, Lee JS, Lai WK, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect 2005; 51: 98–102.
- 18. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected (WHO/2019‐nCoV/clinical/2020.4). Updated 13 Mar 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (viewed Mar 2020).
- 19. Zhao JP, Hu Y, Du RJ, et al. Expert consensus on the use of corticosteroid in patients with 2019‐nCoV pneumonia] [Chinese]. Zhonghua Jie He He Hu Xi Za Zhi [Chinese Journal of Tuberculosis and Respiratory Medicine] 2020; 43: E007.
- 20. Mo Y, Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J Antimicrob Chemother 2016; 71: 3340–3350.
- 21. Haviernik J, Štefánik M, Fojtíková M, et al. Arbidol (Umifenovir): a broad‐spectrum antiviral drug that inhibits medically important arthropod‐borne flaviviruses. Viruses 2018; 10: E184.
- 22. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ 2020 Feb 19; 19(368): m606.
- 23. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; https://doi.org/10.1016/s2213-2600(20)30076-x [Epub ahead of print].
- 24. Quispe‐Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus‐associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med 2010; 36: 33–41.
- 25. Peiris JSM, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 2003; 361: 1767–1772.
- 26. Park JJ, Wong DK, Wahed AS, et al. Hepatitis B virus‐specific and global T‐cell dysfunction in chronic hepatitis B. Gastroenterology 2016; 150: 684–695.
- 27. Lu H. Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Biosci Trends 2020; 14: 69–71.
- 28. Chen J, Xi XH, Liu P, et al. [Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia] [Chinese]. Zhōnghuá chuánrǎn bìng zázhì [Chinese Journal of Infectious Diseases] 2020; https://doi.org/10.3760/cma.j.cn311365-20200210-00050 [Epub ahead of print].





Abstract
Objectives: To assess the efficacy of corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19).
Design, setting: Observational study in the two COVID‐19‐designated hospitals in Wuhu, Anhui province, China, 24 January – 24 February 2020.
Participants: Thirty‐one patients infected with the severe acute respiratory coronavirus 2 (SARS‐CoV‐2) treated at the two designated hospitals.
Main outcome measures: Virus clearance time, length of hospital stay, and duration of symptoms, by treatment type (including or not including corticosteroid therapy).
Results: Eleven of 31 patients with COVID‐19 received corticosteroid treatment. Cox proportional hazards regression analysis indicated no association between corticosteroid treatment and virus clearance time (hazard ratio [HR], 1.26; 95% CI, 0.58–2.74), hospital length of stay (HR, 0.77; 95% CI, 0.33–1.78), or duration of symptoms (HR, 0.86; 95% CI, 0.40–1.83). Univariate analysis indicated that virus clearance was slower in two patients with chronic hepatitis B infections (mean difference, 10.6 days; 95% CI, 6.2–15.1 days).
Conclusions: Corticosteroids are widely used when treating patients with COVID‐19, but we found no association between therapy and outcomes in patients without acute respiratory distress syndrome. An existing HBV infection may delay SARS‐CoV‐2 clearance, and this association should be further investigated.