The maintenance of sustainable low cost physical distancing and enhanced hygiene may decrease the number and severity of cases
It is estimated that about two‐thirds of cases of coronavirus disease 2019 (COVID‐19) exported from China between 1 and 13 January 2020 were undetected globally.1 Most of these exported cases were mild and were only detected after several hundred cases had accumulated and severe or fatal cases were recognised 5–8 weeks later, as likely occurred in the COVID‐19 outbreaks in Iran, South Korea, Italy and Seattle, United States.2
The spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission globally has been very rapid. The basic reproduction number (R0) is estimated at between 2 and 3.3,4 The mode of transmission is thought to be droplet and contact infection, although opportunistic or close range airborne infection may be involved.4
The transmission dynamics of the early cases of COVID‐19 were significantly different to those during the severe acute respiratory syndrome (SARS) epidemic in 2003. In particular, the proportion of COVID‐19 cases from health care settings was low and the proportion with no known risk exposures was high.4 Another significant factor is that viral loads in nasopharyngeal and respiratory secretions are highest soon after symptom onset in patients with COVID‐195 compared with a peak of around 10 days in patients with SARS,6 making transmission before entering health care facilities and in the pre‐symptomatic phase more likely.7
Even though the understanding of transmission dynamics is at an early stage, they do suggest that the stepwise introduction of stringent measures will be necessary to control this epidemic and highlight the importance of early community control. Australia and other countries have experienced a first wave of disease and managed to effect a decline in cases.8
Quarantine; city lockdowns; complete childcare, school, university and workplace closures; and cancellation of mass gatherings and events have a significant social and economic impact and were not often implemented until significant transmission was confirmed — when they may be less effective. Countries are now challenged with identifying which of these various controls can be relaxed to allow some routine societal and economic activities to return. However, there are low cost, sustainable interventions that may be maintained over what may be many years of continued mitigation9 (Box 1). These low cost enhanced hygiene and physical distancing measures are applicable pre‐emptively before confirmation of local community transmission or where transmission of SARS‐CoV‐2 appears to be under control.
The purpose of these interventions is to slow the transmission of disease and limit the impact on health services, particularly on hospitals and intensive care units, to ensure access to high level care when needed.
The interventions are based on the following assumptions, which require further exploration:
- community‐wide SARS‐CoV‐2 transmission may be occurring undetected or may only be recognised after containment is no longer feasible;
- interventions implemented after community‐wide transmission is detected will be less effective;
- reduction of the force of infection, particularly early, will delay the epidemic peak, blunt the epidemic peak, spread cases over a longer time, and help limit the potential for critical care services to be overwhelmed, which may be lifesaving;12,13
- low cost sustainable interventions will assist in the relaxation of more economically costly interventions, and
- enhanced hygiene and physical distancing interventions should:
- ▸decrease the total number of cases per week; and
- ▸decrease the severity of cases through reducing viral inocula.
Box 2 illustrates the concept of limiting the peak in cases so that health services are less likely to be overwhelmed and there is less unmet health service need. Unmet need may include inability to admit patients to a hospital or to provide hospitalised patients in critical condition access to intensive care. Interventions to reduce infection lead to longer but less peaked epidemics. A slower evolution in the epidemic also allows time for health care staff to provide better care, for recovery of infected health care workers, for learning and adapting to the evolving situation by administrators, and for vaccines and treatments to be developed. This principle is validated in simulations for influenza14 and appears to be validated with the reduction in COVID‐19 cases in Australia and the relative lack of overburden on clinical services.8
Measures to decrease the number and severity of cases
Pre‐emptive and ongoing maintenance of low cost interventions (such as enhanced hygiene and physical distancing measures) (Box 1) may not only decrease the total number of cases but may also decrease the severity of cases.
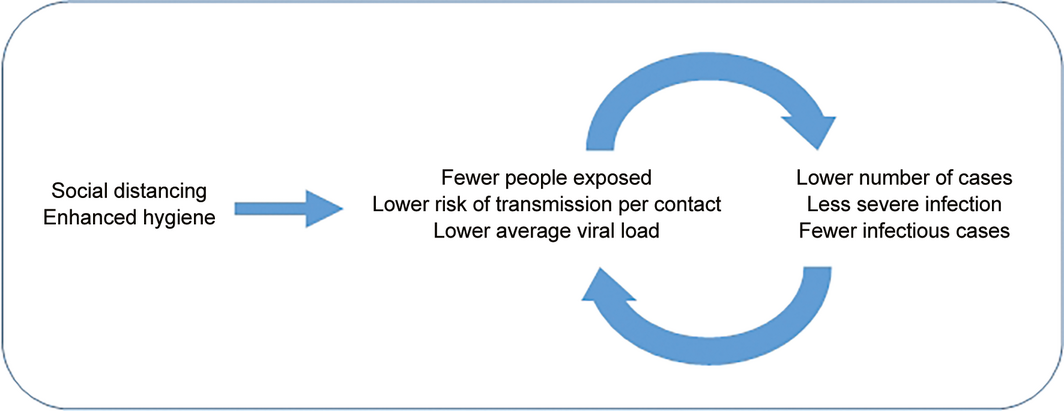
The R0 is the average number of secondary cases of an infectious disease that arise from cases in a totally susceptible population and reflects the epidemic potential of a pathogen.15R0 is a function of the number of contacts an infectious person has, the risk of transmission per contact, and the duration of infectiousness.
Physical distancing mostly acts on the first factor by reducing the number of contacts each person makes. Hygiene measures mostly act on the second factor, as they reduce the risk of transmission if a contact occurs. It is difficult to disentangle the effectiveness of the multiple control measures implemented in pandemic‐affected areas. The World Health Organization–China Joint Mission on COVID‐19 determined that widespread community transmission and outbreaks occurred in Wuhan before the implementation of comprehensive control measures.4 However, in other parts of China, community transmission has been limited and after public gatherings were cancelled and people were restricted to their homes, most transmission occurred in families. For example, among 344 clusters involving 1308 cases (out of a total 1836 cases reported) in Guangdong Province and Sichuan Province, 78–85% have occurred in families.4
Community‐wide interventions may decrease the average viral exposure dose encountered in the community. People exposed to a higher viral dose (inoculum) are more likely to become infected and have more severe disease. Animal models for other coronavirus infections demonstrate that increased viral inocula lead to more severe disease and higher viral loads in the lungs and other organs and fluids.16 The SARS outbreak in Amoy Gardens, Hong Kong, in 2003 provided evidence that patients with presumed higher exposure to the index case had higher nasopharyngeal viral loads and more severe illness.17 SARS‐CoV‐2 cases with more severe disease have been found to have around 60 times higher viral load than those with mild disease.18 Modelling of the 2009 influenza pandemic also supported a hypothesis that severe illness was due to a higher infectious dose of the virus mediated by the number of simultaneous infectious contacts.19 Viral loads in severe patients with Middle East respiratory syndrome (MERS) were higher than those in a mild group, and the patients in the severe group had more prolonged viral shedding in respiratory secretions, beyond 21 days after the onset of symptoms, whereas viral RNA was no longer detected by 21 days in the mild group.20
Therefore, it is proposed that early measures that lower the number of contacts, the likelihood of transmission, and average viral infective dose in an area of transmission may have a multiplier effect leading to fewer cases and fewer severe cases that are less infectious. Maintaining the early reduction of the R0 would result in fewer cases overall and have a significant negative multiplier effect on the overall impact of the epidemic, including the number of deaths (Box 3). The higher case fatality rate in Wuhan, compared with other provinces in China, may partially relate to health care resource availability and shortages in the face of overwhelming community transmission as well as greater severity of disease due to higher infection doses.12,17 These interventions will be particularly important for people over 60 years of age and those with underlying medical conditions.
The costs of intervention
The suite of low cost interventions, other than a working from home policy, is unlikely to affect work productivity and may provide the community with some reassurance that all is being done to prevent the epidemic and that maintenance of the low cost measures may allow earlier opening of some workplaces. WHO is supportive of pre‐emptive interventions to prevent COVID‐19 in workplaces.21 Some may see it as being overreaching, but thus far, communities seem to voluntarily adopt low cost interventions, and acceptance may be enhanced through consultation and trust building.22,23
Influenza co‐benefits
For regions approaching their influenza season, optimal prevention and control of seasonal influenza, such as vaccination, in the face of potential COVID‐19 cocirculation is also crucial to minimise the double burden on health services. The measures discussed here (enhanced hygiene and physical distancing) are also effective against influenza, resulting in potential co‐benefits for both pathogens. Early indications from Flutracking.net (https://info.flutracking.net/reports-2/australia-reports) indicate that physical distancing and hygiene enhancements have markedly decreased influenza‐like illness in Australia.
Limitations
While physical distancing and enhanced hygiene interventions in Australia appear to be working, the evidence on the effectiveness of individual interventions in preventing COVID‐19 is not yet available. However, there is evidence from observational and simulation studies for the effectiveness of physical distancing measures in controlling seasonal influenza.13 Other measures, such as hand hygiene and cleaning surfaces, have a long history of use in infection prevention and control.24 Despite the lack of robust evidence of effectiveness for these measures, their relative low cost means that there is little harm and much potential benefit in maintaining and optimising them.
We have made no recommendations in regard to masks. The use of masks outside of health settings is controversial and it is important that medical grade masks not be diverted from health care supplies. Nevertheless, surgical masks are protective of large droplet spread, have about half the effectiveness of N95 masks for small droplet transmission, and are suggested to be cost‐saving in some modelled pandemic influenza scenarios.25 The use of masks may have a role in the community setting if there are adequate supplies.10 There is evidence suggesting that community use of masks may have reduced the risk of contracting SARS.26 It is clear that masks should be used in households caring for patients with COVID‐19 at home. Policy development and scientific review of the literature on community use of masks is very dynamic at this time. The US Centers for Disease Control and Prevention has made a recent recommendation that cloth masks be used at the community level and many recent reviews have come to divergent conclusions about the usefulness and risks of community mask use.27,28,29,30 Coherent policy development in this space will rely on transparently articulating the scientific evidence on community mask use with a public conversation on the potential risks in implementation.
The interventions discussed here should be tailored to individual settings and communities, in partnership with members of those communities. In particular, these interventions should be adapted to the unique circumstances of groups, such as Indigenous communities; vulnerable groups, including homeless populations; and culturally and linguistic diverse communities.
Conclusion
SARS‐CoV‐2 continues to disseminate globally and there are likely to be recurrent waves of infection into the foreseeable future. We would argue that these low cost interventions, although formulated at an earlier stage of the epidemic, have increasing relevance. They will protect against the emerging concern for pre‐symptomatic transmission and their optimisation will better enable the more restrictive and economically damaging constraints to be relaxed.7
Box 1 – Low cost hygiene and physical distancing interventions
|
Settings |
Interventions |
||||||||||||||
|
|
|||||||||||||||
|
Workplace |
|
||||||||||||||
|
School |
|
||||||||||||||
|
Commercial, entertainment and transport |
|
||||||||||||||
|
Household |
|
||||||||||||||
|
All households |
|
||||||||||||||
|
Households with ill members |
|
||||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019. * Ill person refers to someone with symptoms of respiratory illness or fever, who is not yet under investigation for COVID‐19 but could be an unrecognised case. † This could be costly unless used judiciously while awaiting exclusion of COVID‐19 in the suspected case and should be introduced based on likelihood of local transmission. ‡ Evidence that low temperature and low humidity in air‐conditioned environments may enhance the survival of coronaviruses such as severe acute respiratory syndrome (SARS).11 § When international travel restrictions are lifted, sites such as the Centers for Disease Control and Prevention travel risk assessment site may be useful (https://www.cdc.gov/coronavirus/2019-ncov/travelers/map-and-travel-notices.html). |
|||||||||||||||
Box 2 – Intended impact of enhanced hygiene and physical distancing measures on the coronavirus disease 2019 (COVID‐19) pandemic*
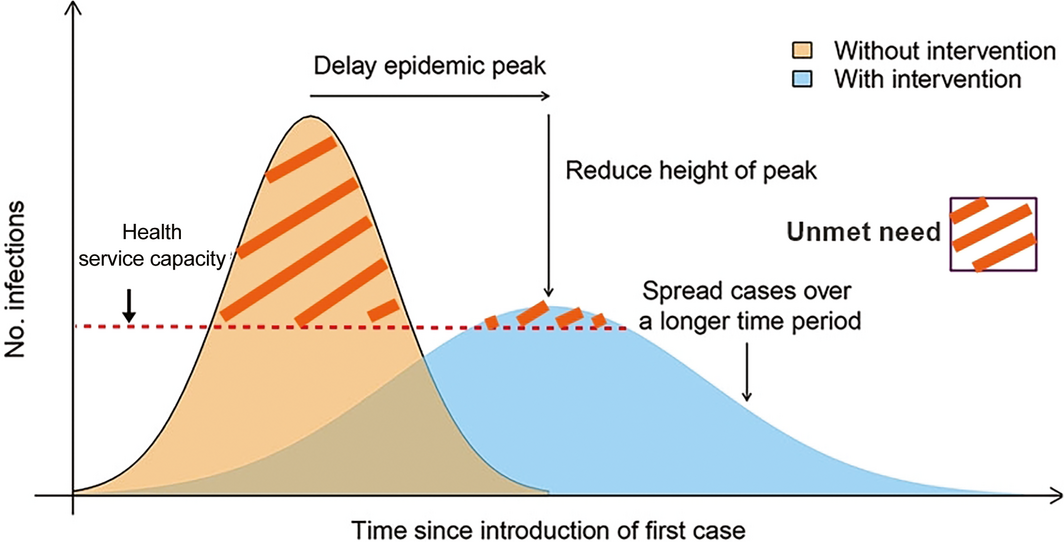
*Figure adapted from Fong et al.13
Provenance: Not commissioned; externally peer reviewed.
- 1. Bhatia S, Imai N, Cuomo‐Dannenburg G, et al. Report 6: relative sensitivity of international surveillance. London: Imperial College London; 2020. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-international-surveillance-21-02-2020.pdf (viewed Apr 2020).
- 2. MacIntyre CR. Global spread of COVID‐19 and pandemic potential. Global Biosecurity 2020; 1.
- 3. Liu Y, Gayle AA, Wilder‐Smith A, Rocklöv J. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med 2020; 27: taaa021.
- 4. World Health Organization. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID‐19). WHO, 2020; pp. 1–40. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (viewed Apr 2020).
- 5. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382: 1177–1179.
- 6. Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 2003; 361: 1767–1772.
- 7. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med 2020; https://doi.org/10.1038/s41591-020-0869-5.
- 8. Department of Health; COVID‐19 National Incident Room Surveillance Team. Weekly epidemiological report — COVID‐19, Australia: epidemiology report 10. Commun Dis Intell 2020; 44; https://doi.org/10.33321/cdi.2020.44.30.
- 9. Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science 2020; eabb5793.
- 10. Wein LM, Atkinson MP. Assessing infection control measures for pandemic influenza. Risk Anal 2009; 29: 949–962.
- 11. Chan KH, Peiris JS, Lam SY, et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol 2011; 2011: 734690.
- 12. Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID‐19 mortality and health‐care resource availability. Lancet Glob Health 2020; 8: pe480.
- 13. Fong MW, Gao H, Wong JY, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings — social distancing measures. Emerg Infect Dis 2020; 26.
- 14. Kelso JK, Milne GJ, Kelly H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 2009; 9: 117.
- 15. Fine P. Introduction: the basics — infections, transmission and models. In: Vynnycky E, White R. An introduction to infectious disease modelling. UK: Oxford University Press, 2010.
- 16. Douglas MG, Kocher JF, Scobey T, et al. Adaptive evolution influences the infectious dose of MERS‐CoV necessary to achieve severe respiratory disease. Virology 2018; 517: 98–107.
- 17. Chu CM, Cheng VCC, Hung IFN, et al. Viral load distribution in SARS outbreak. Emerg Infect Dis 2005; 11: 1882–1886.
- 18. Liu Y, Yan L, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis 2020; https://doi.org/10.1016/s1473-3099(20)30232-2. [Epub ahead of print]
- 19. Paulo AC, Correia‐Neves M, Dominguos T, et al. Influenza infectious dose may explain the high mortality of the second and third wave of 1918–1919 influenza pandemic. PLoS ONE 2010; 5: e11655.
- 20. Oh MD, Park WB, Choe PG, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med 2016; 375: 1303–1305.
- 21. World Health Organization. Getting your workplace ready for COVID‐19. WHO, 2020. https://www.who.int/docs/default-source/coronaviruse/getting-workplace-ready-for-covid-19.pdf (viewed Apr 2020).
- 22. Braunack‐Mayer AJ, Street JM, Rogers WA, et al. Including the public in pandemic planning: a deliberative approach. BMC Public Health 2010; 10: 501.
- 23. Prati G, Pietrantoni L, Zani B. Compliance with recommendations for pandemic influenza H1N1 2009: the role of trust and personal beliefs. Health Educ Res 2011; 26: 761–769.
- 24. Best M, Neuhauser D. Ignaz Semmelweis and the birth of infection control. Qual Saf Health Care 2004; 13: 233–234.
- 25. Mukerji S, MacIntyre CR, Newall AT. Review of economic evaluations of mask and respirator use for protection against respiratory infection transmission. BMC Infect Dis 2015; 5: 413.
- 26. Public Health England. The use of facemasks and respirators during an influenza pandemic: scientific evidence base review. London: Crown, 2014 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/316198/Masks_and_Respirators_Science_Review.pdf (viewed Apr 2020).
- 27. Leung NHL, Chu DLW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; https://doi.org/10.1038/s41591-020-0843-2.
- 28. National Academies of Sciences, Engineering, Medicine. Rapid expert consultation on the effectiveness of fabric masks for the COVID‐19 pandemic; 8 April, 2020; https://www.nap.edu/read/25776/chapter/1 (viewed Apr 2020).
- 29. Greenhalgh T, Schmid MB, Czypionka T, et al. Face masks for the public during the covid‐19 crisis. BMJ 2020; 369: m1435.
- 30. Howard J, Huang A, Li Z, et al. Face masks against COVID‐ 19: an evidence review [preprint]. Preprints 2020; https://10.20944/preprints202004.0203.v1.





We thank Kirsten Williamson for her thoughtful review of the draft manuscript.
No relevant disclosures.