Until recently, analgesic medications containing less than 30 mg codeine per dosage unit were available over the counter in Australia.1 Codeine‐related hospital presentations placed an increasing economic burden on the Australian health care system;2 in response, the Therapeutic Goods Administration re‐scheduled all codeine‐containing products as prescription‐only medicines from 1 February 2018.3
Our clinical toxicology unit undertook a retrospective review of codeine‐related presentations (deliberate self‐poisonings and recreational exposures) from 1 February 2017 to 31 January 2019; that is, the 12 months preceding and the 12 months following codeine rescheduling. Our unit manages all patients who present with poisoning to the Princess Alexandra Hospital in Brisbane. Data are prospectively entered into a purpose‐built database that we searched for codeine‐related presentations; we examined patient medical records for ingestion information, clinical features, treatment, and complications. The statistical significance of differences in proportions was assessed in Fisher exact tests. Our study received ethics approval from Metro South Health Human Research Ethics Committee (reference, HREC/14/QPAH/308).
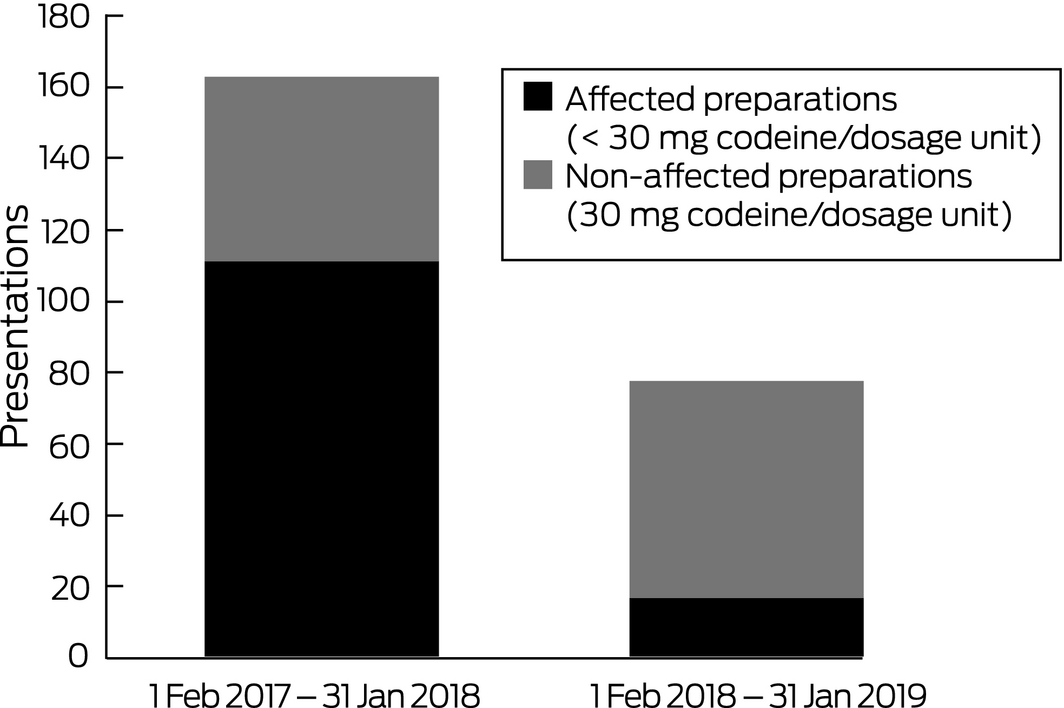
A total of 2235 patients presenting with poisoning were managed by our unit during the 12 months preceding the rescheduling of codeine, and 2516 during the subsequent 12 months, a 13% increase. However, the number of codeine‐related presentations was 53% lower during the second period: there were 163 presentations before and 77 presentations after rescheduling (P < 0.001). This decline followed the number of codeine‐related presentations rising from 133 in 2015 to 159 in 2016. The numbers of presentations involving 30 mg codeine products, the status of which was unaffected by rescheduling, were similar for the two periods (52 before, 60 after rescheduling; P = 0.92). In contrast, the number of presentations involving codeine products affected by the change (< 30 mg) was 85% lower after rescheduling (111 presentations before, 17 presentations after rescheduling; P < 0.001; Box). The decline in codeine‐related presentations was not associated with a rise in alternative opioid‐related presentations (185 alternative opioid‐related presentations before, 178 after rescheduling; P = 0.13).
The median amount of codeine ingested was similar in both periods: 200 mg (interquartile range [IQR], 130–390 mg; range 6–1800 mg) before and 270 mg (IQR, 120–600 mg; range, 24–2280 mg) after rescheduling. About 90% of the products ingested in each period were paracetamol co‐formulations. Acetylcysteine was administered to patients during 47 presentations before and 17 presentations after rescheduling.
Severe toxicity was rare; naloxone reversal of opioid toxicity was infrequent (14 cases before, 10 after rescheduling) and almost exclusively related to co‐ingestion of other opioids. One patient died in the 12 months prior to codeine rescheduling, after delayed presentation and with established paracetamol‐induced hepatic failure. Two patients died during the 12 months after rescheduling; one death was secondary to a massive propranolol co‐ingestion, the other followed delayed presentation with codeine‐induced opioid toxicity, resulting in severe hypoxic brain injury.
Our study was limited by its single centre, retrospective design. Patients may have presented to other hospitals after codeine was rescheduled, but the total number of poisoning presentations to our unit was higher during the period after rescheduling.
In summary, rescheduling of codeine‐containing products was associated with a significant reduction in codeine‐related presentations to our clinical toxicology unit without the number of alternative opioid‐related presentations increasing.
Received 14 June 2019, accepted 21 July 2019
- 1. Mill D, Johnson JL, Cock V, et al. Counting the cost of over‐the‐counter codeine containing analgesic misuse: a retrospective review of hospital admissions over a 5 year period. Drug Alcohol Rev 2018; 37: 247–256.
- 2. Roberts DM, Nielsen S. Changes for codeine. Aust Prescr 2018; 41: 2–3.
- 3. Therapeutic Goods Administration. Update on the proposal for the rescheduling of codeine products [media release]. 20 Dec 2016. https://www.tga.gov.au/media-release/update-proposal-rescheduling-codeine-products (viewed July 2019).





No relevant disclosures.