The known: Excisional biopsy is recommended for diagnosing invasive melanoma. Some US studies have reported high rates of shave and saucerisation techniques for diagnosing invasive melanoma, raising concerns that tumour depth assessment and microstaging may be compromised.
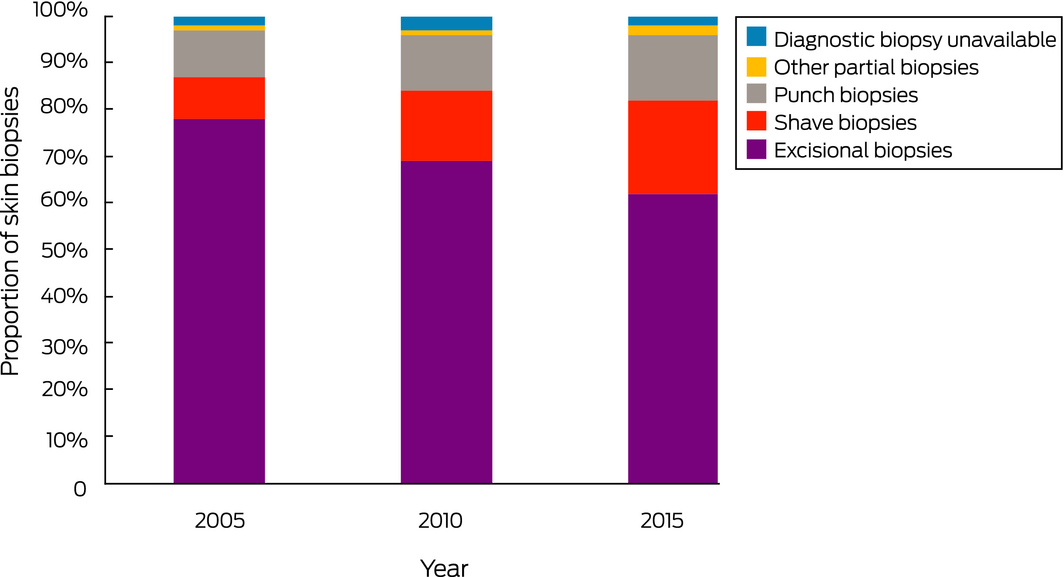
The new: Shave biopsies are increasingly used to diagnose invasive melanomas, their proportion of all melanoma biopsies rising from 9% in 2005 to 20% in 2015. Shave biopsies are associated with high rates of base transection (54%) and T‐upstaging (12%), and underestimate tumour thickness by a mean 0.25 mm.
The implications: Excisional biopsy remains the most appropriate diagnostic biopsy technique for invasive melanoma.
The aim of diagnostic skin biopsy is to accurately detect or exclude melanoma and to accurately stage the primary tumour in order to inform therapy planning. There are critical differences in the ability of the various methods of complete and partial biopsy to achieve this outcome,1 and melanoma guidelines in Australia, the United States and Europe advocate complete excisional biopsy.2,3,4 However, the decision to completely remove or to sample a lesion depends on the individual case.
Punch and shave biopsies (superficial shave and shave saucerisation) are the most widely employed types of partial biopsy. Punch biopsies are usually intended to sample and diagnose large, clinically obvious melanomas (high false negative rates make them unsuitable for diagnosing clinically equivocal lesions),1 whereas the aim with many shave biopsies is to diagnose and completely remove the lesion, thereby allowing the planning of definitive therapy.2
According to Australian melanoma clinical guidelines,2 partial biopsies can be appropriate for patients with significant comorbid conditions, large lesions, or lesions in acral or other locations where excisional biopsy could lead to unwanted functional or cosmetic outcomes. However, recent studies have reported high rates of shave biopsy, suggesting they are not restricted to such cases.5,6,7,8,9,10,11 Population trends in the use of punch and shave biopsy techniques have not been formally examined.
While shave and saucerisation biopsy techniques have been promoted as more accessible and less expensive than excision, they are associated with microstaging inaccuracy.1,8 This is more likely with shave biopsy than excisional biopsy (odds ratio, 2.3; 95% confidence interval [CI], 1.5–3.6), and the odds of significant inaccuracy increase by 80% (95% CI, 40–140%) for each 1 mm increase in tumour thickness.1
Although it may be appropriate for in situ melanomas, shave biopsy can transect the base of invasive melanomas.1,7,10 Base transection may lead to inaccurate initial assessment of tumour depth, with negative implications for microstaging, planning of therapy, and determining the prognosis. Further, tumour base transection can lead to irretrievable destruction of deeper tumour tissue through inflammation or the effects of the haemostasis method employed, and thereby to the loss of information useful for prognosis. Base transection rates for shave biopsy of 7%, 9%, 37%, 64%, 65%, and 68% have been reported.6,7,8,9,10,11
Accurate assessment of tumour thickness is critical for determining not only whether sentinel lymph node biopsy should be offered, but also for assigning prognostic stage III groupings according to the revised American Joint Committee on Cancer (AJCC) staging system;12 this in turn determines access to adjuvant therapies that have recently been found to significantly reduce the risk of melanoma recurrence in patients with stage III disease.13,14
We assessed changes in the choice of skin biopsy technique for assessing invasive melanoma over a ten‐year period in Victoria, and examined the impact of partial biopsy technique on the accuracy of microstaging.
Method
We undertook a retrospective, cross‐sectional review of Victorian Cancer Registry (VCR) data on invasive melanoma histologically diagnosed in Victoria during 2005, 2010 and 2015. The VCR has collected information on all cancer diagnoses for Victorian residents since 1982. Hospitals, pathology laboratories, and cancer screening registers are legally obliged to report cancer diagnoses to the VCR, ensuring complete population coverage.15
Data selection
Our database did not contain information on patients with in situ melanoma. After excluding data for 768 patients for whom data on tumour thickness were missing, the primary tumour was unknown, or who had metastatic disease at the time of diagnosis, we randomly selected 400 patients for each of the three years examined, stratified by final tumour thickness: 200 patients with thin melanoma (< 1.0 mm), 100 with intermediate melanoma (1.0–4.0 mm), and 100 with thick melanoma (> 4.0 mm). The pathology records of sampled patients were reviewed for data on diagnostic biopsy type, anatomic location of the melanoma, tumour transection, presence of residual melanoma on wide local excision (WLE), melanoma final thickness, and the type of clinician who undertook the biopsy (determined by cross‐referencing their name and principal place of practice as recorded in the VCR with publicly available online information).
Mean tumour thickness underestimation was defined as the difference between final Breslow thickness and thickness determined by diagnostic biopsy. T‐upstaging was defined as an increase in T‐category according to the AJCC staging system (8th edition).12
Statistical analysis
We assessed associations (expressed as odds ratios [ORs]) between biopsy type (excisional, partial) and year, residual melanoma, melanoma thickness, and T‐upstaging in logistic regression analyses. We assessed associations (expressed as relative risk ratios [RRRs], which can be interpreted as ORs) between biopsy type (excisional, shave, punch) and year in multinomial logistic regression analyses. All analyses were conducted in Stata 15 (StataCorp).
Ethics approval
Ethics approval for the study was granted by the Alfred Hospital Human Research Ethics Committee (reference, 78/17).
Results
In Victoria, 2519 people were diagnosed with invasive melanoma in 2005, 2691 in 2010, and 3067 in 2015. The randomly selected 1200 patients included 11–13% of cases of thin melanoma, 12–17% of intermediate tumours, and 51–65% of thick melanomas by year. There had been 833 excisional biopsies (69% of patients), 337 partial biopsies (28%), and 30 cases (3%) in which WLE specimens, but no diagnostic biopsy, were available for assessment. Partial diagnostic biopsies included 143 punch (42%), 175 shave (52%), seven incisional (4%), and 12 curettage procedures (2%) (Box 1). Given their low numbers, incisional partial biopsies and curettes were excluded from our analyses, except as noted.
Partial biopsies as a proportion of all diagnostic procedures increased from 20% in 2005 to 36% in 2015 (OR, 2.32; 95% CI, 1.69–3.20; P < 0.001). For thin melanomas, the partial biopsy proportion increased from 26% to 42% (OR, 2.16; 95% CI, 1.41–3.30; P < 0.001); for intermediate thickness melanomas, it increased from 16% to 38% (OR 3.22; 95% CI, 1.65–6.29; P = 0.001); for thick melanomas, it increased from 13% in 2005 to 24% in 2015 (OR, 2.11; 95% CI, 1.01–4.44; P = 0.048) (Box 2).
Shave biopsies comprised 9% of all diagnostic procedures in 2005, rising to 20% in 2015 (RRR, 2.77; 95% CI, 1.81–4.23; P < 0.001). The proportion of punch biopsies increased from 10% in 2005 to 14% in 2015 (RRR, 1.75; 95% CI, 1.13–2.71; P = 0.012) (Box 3). For thin melanomas, the proportion of shave biopsies doubled from 15% in 2005 to 31% in 2015; 6% of all intermediate thickness melanomas in 2005 were diagnosed by shave biopsy, rising to 14% in 2015 (Box 2).
Eighty‐six of 175 shave biopsies (49%) during the three years examined were performed by dermatologists, 69 (39%) by general practitioners, and 14 (8%) by surgeons. The proportion of shave biopsies among all procedures by dermatologists increased from 29% in 2005 to 45% in 2015, and for general practitioners from 6% to 19%; for surgeons, the proportion was consistently below 10% (Box 4).
Shave biopsies without tumour base transection
Of 175 diagnostic shave biopsies, 81 (46%) did not transect the tumour base; of these, there was no residual melanoma on WLE in 26 cases (32%) and WLE was not available for assessment in 29 cases (36%). Of the 26 cases (32%) with residual melanoma on WLE, 22 were thin melanomas, three intermediate melanomas, and one a thick melanoma. In seven of 81 cases of shave biopsy with peripheral (but not tumour base) transection (9%), the tumour was later T‐upstaged (five thin, one intermediate, one thick tumour).
Shave biopsies with tumour base transection
Of 175 diagnostic shave biopsies, 94 (54%) transected the tumour base; residual melanoma was identified by WLE in 65 cases (69%), and 14 tumours (15%) were later T‐upstaged. Fifty‐eight of 130 thin melanomas (45%) diagnosed with shave biopsy were transected at the base, including 29 of 83 very superficially invasive melanomas (≤ 0.5 mm; 35%). While no thin tumours diagnosed by excisional biopsy were T‐upstaged, three of 58 thin tumours with base transection on shave biopsy (5%) were T‐upstaged. Twenty‐one of 58 thin melanomas (36%) had no assessable tumour on WLE after base transection. Twenty‐four of 32 intermediate thickness melanomas (75%) were transected at the base, seven of which (29%) were T‐upstaged and three (13%) had no assessable tumour on WLE. Of the 13 thick melanomas diagnosed by shave biopsy, 12 (92%) were transected at the base, four of which were T‐upstaged and five had no assessable residual tumour on WLE (Box 5).
Shave biopsy and T‐upstaging
The overall rate of T‐upstaging for shave biopsies was 12% (21 of 175). The proportion of tumours that were T‐upstaged increased with tumour thickness: eight of 130 thin melanomas (6%), eight of 32 intermediate melanomas (25%; v thin: OR, 4.5; 95% CI, 2.5–8.1; P < 0.001), and five of 13 thick melanomas (38%; v thin: OR, 9.1; 95% CI, 4.5–18.0; P < 0.001). In contrast, 25 of 833 excisional biopsies (3%) were associated with residual melanoma on subsequent WLE; of these, eight (1%) were T‐upstaged.
Tumour thickness may be underestimated in shave biopsies as the result of base transection or unrepresentative sampling of a large lesion. For shave biopsies (with or without base transection), thickness was underestimated to a greater extent for thick melanomas (mean underestimation, 2.15 mm; standard deviation [SD], 4.53 mm) than for thin melanomas (mean underestimation, 0.06 mm; SD, 0.16 mm). For shave biopsies with base transection, tumour thickness was underestimated by a mean 0.46 mm (SD, 1.82 mm) (Box 6).
Discussion
This is the first population‐based study to examine changes in rates of partial skin biopsy for diagnosing invasive melanoma. We found that partial biopsy has been increasingly employed for this purpose in Victoria; in particular, the proportion of shave biopsies increased from 9% of all skin biopsies in 2005 to 20% in 2015, despite guideline recommendations.2,3,4 Although our data are state‐specific, they may reflect a wider change in practice; some US institutions have reported that shave biopsy and saucerisation is used for 18–61% of biopsies for invasive melanoma.6,7,9,10,11
Our findings suggest that shave biopsy is increasingly being applied when diagnosing invasive melanoma,8,11,16,17 not just in situ lesions or those with a low index of suspicion. Other possible reasons for the change in practice are that shave biopsies can be performed quickly, are inexpensive, require less equipment, and do not require re‐scheduling patients as often as excisional biopsy.16,18,19,20 These features reduce the barrier to biopsy and minimise the risk that melanomas with a low index of suspicion will be missed or delayed, particularly by dermatologists, who see a large number of patients at high risk, and general practitioners, who are responsible for most melanoma diagnoses in community practice. The advantages of the shave technique must, however, be balanced against its deficiencies in accurately microstaging an invasive tumour.
Advocates of shave biopsy and saucerisation argue that they provide adequate depth assessment for superficially invasive melanomas.8,16 We found a high rate of base transection (45%) for shave biopsy of thin invasive melanomas (< 1.0 mm); even for very superficially invasive melanomas (≤ 0.5 mm thick), base transection was frequent (35%). It has been suggested that doctors with training and experience are more able to select lesions for shave biopsy appropriately and are less likely to transect their bases,21 but we found that base transection was common among both dermatologists and general practitioners (data not shown).
Base transection is a particular problem when the tumour thickness is close to the 1 mm threshold for sentinel lymph node biopsy, thereby creating a dilemma for management and impeding the accuracy of further staging. This is especially pertinent because the new (eighth) edition of the AJCC staging system takes tumour thickness into account for stratifying stage III categories. Sentinel node biopsy improves prognostication and enables access to adjuvant therapies for stage III melanoma, which improve patient outcomes by reducing the risk of melanoma recurrence.13,14 Accurate staging is therefore crucial if all therapeutic options for patients are to be available.
We also found that shave biopsy was increasingly used to diagnose intermediate thickness tumours, and that the base was transected in 75% of such cases. As ongoing adjuvant treatment trials include patients with stage IIB and C tumours classified as T3b (2.01–4.0 mm with ulceration) or T4 (> 4.0mm),22 the 2.0 mm and 4.0 mm thickness cut‐offs are critical for management decisions. While inaccurate tumour microstaging may not have directly affected patient outcomes in the past, the choice of biopsy technique may influence patient outcomes in the era of effective adjuvant therapy.
Base transection rates of more than 60% have been reported in three other studies.6,10,11 We found that the rate increased with tumour thickness (45%, 75% and 92% for thin, intermediate, and thick melanomas). An important question is whether shave biopsy is inferior to excisional biopsy with regard to microstaging, as has been suggested.1,6,7,23,24 We found that with excisional biopsies 1% of tumours were T‐upstaged, compared with 12% with shave biopsies. Other studies have reported T‐upstaging rates of 2–3% with excisional biopsy and of 3–12% with shave biopsy;6,8,9,16 one reported that eight of 114 thin (T1) tumours (7%) were T‐upstaged following shave biopsy,16 similar to our rate of 5% for thin tumours. The rate of T‐upstaging and the underestimation of tumour thickness in our study were each relatively low for thin melanomas, so the risk of understaging is likely to be small for unsuspected superficial invasion detected after a shave biopsy.
Shave biopsy with base transection was associated with underestimation of tumour thickness by a mean 0.46 mm, and underestimation was greater for thick than thin melanomas. These data refer to tumours for which there was assessable residual tumour, and partial or complete loss of residual tumour in other cases may have reduced the mean underestimation.
Current guidelines2 recommend that partial biopsy can be appropriate for large lesions and tumours on the head, distal lower limb, and where excisional biopsy with primary closure cannot be achieved. Shave biopsy may be appropriate for in situ melanomas. However, one large study found that the odds of false negative misdiagnoses, while not as high as for punch biopsy, are still significantly greater for shave than excisional biopsies (OR, 2.6; P = 0.02; adjusted OR, 4.5, P = 0.002), with most adverse outcomes associated with involved margins and an incorrect initial pathologic diagnosis of benign melanocytic lesion.1 Given the increasing use of the shave technique and high rates of margin involvement, the frequency of adverse outcomes is therefore likely to increase.
The decision for partial biopsy may be influenced by the index of suspicion, comorbid conditions, and the risk of negative functional or cosmetic outcomes in difficult locations. Our data suggest that shave biopsy is increasingly used for assessing melanomas that do not meet these criteria or have clinical features associated with invasive melanoma, including elevation, induration, bleeding, ulceration, and dermoscopic features such as blue or grey colour, atypical vessels, white streaks, or regression features. We advocate excising such melanomas for the initial diagnosis.
Limitations
We analysed population data, enabling more accurate assessment of biopsy practices over time. Selection bias was minimised by randomly selecting and stratifying patients according to final tumour thickness. Limitations of our study included its retrospective nature, which limited our assessing clinician intent to completely include tumour depth in a biopsy. Further, a centralised pathology review to reduce errors of interpretation, to obtain information about tumour diameter, and to enable estimation of potential tumour loss following base transection was not possible because of the size of the data set.
Conclusion
This population‐based study of invasive melanoma identified a marked increase in the use of shave biopsy in Victoria between 2005 and 2015, associated with a substantial rate of tumour base transection and underestimation of tumour thickness. Accurately ascertaining thickness is increasingly important not only for prognosis, but also for decisions about adjuvant therapies and clinical trial opportunities. Where excisional biopsy is readily achievable, it remains the most appropriate diagnostic biopsy technique for assessing invasive melanoma.
Box 1 – Characteristics of 1200 patients diagnosed with invasive melanoma in Victoria during 2005, 2010 and 2015, and of the melanomas and biopsying clinicians, by biopsy type
|
Characteristic |
|
Partial biopsy |
|
||||||||||||
|
Excisional biopsy |
Punch biopsy |
Shave biopsy |
Total |
||||||||||||
|
|
|||||||||||||||
|
Total number of patients |
833 (73%) |
143 (12%) |
175 (15%) |
1151 |
|||||||||||
|
Sex |
|
|
|
|
|||||||||||
|
Men |
514 (75%) |
69 (10%) |
102 (15%) |
685 |
|||||||||||
|
Women |
319 (69%) |
74 (15%) |
73 (15%) |
466 |
|||||||||||
|
Age at diagnosis (years) |
|
|
|
|
|||||||||||
|
< 50 |
158 (80%) |
19 (9%) |
23 (11%) |
200 |
|||||||||||
|
50–70 |
315 (71%) |
49 (11%) |
81 (18%) |
445 |
|||||||||||
|
> 70 |
360 (72%) |
75 (15%) |
71 (13%) |
506 |
|||||||||||
|
Melanoma subtype |
|
|
|
|
|||||||||||
|
Nodular |
75 (75%) |
14 (14%) |
11 (11%) |
100 |
|||||||||||
|
Lentigo maligna |
78 (52%) |
29 (19%) |
44 (29%) |
151 |
|||||||||||
|
Superficial spreading |
283 (68%) |
51 (12%) |
82 (20%) |
416 |
|||||||||||
|
Acral lentiginous |
4 (50%) |
4 (50%) |
0 |
8 |
|||||||||||
|
Desmosplastic |
26 (90%) |
3 (10%) |
0 |
29 |
|||||||||||
|
Not specified |
362 (83%) |
43 (9%) |
38 (8%) |
443 |
|||||||||||
|
Other |
5 (100%) |
0 |
0 |
5 |
|||||||||||
|
Primary tumour site |
|
|
|
|
|||||||||||
|
Head and neck |
186 (67%) |
48 (17%) |
45 (16%) |
279 |
|||||||||||
|
Trunk |
260 (77%) |
24 (7%) |
55 (16%) |
339 |
|||||||||||
|
Upper limb |
215 (79%) |
25 (9%) |
32 (12%) |
272 |
|||||||||||
|
Lower limb |
141 (66%) |
36 (17%) |
36 (17%) |
213 |
|||||||||||
|
Not specified |
31 (78%) |
10 (13%) |
7 (9%) |
78 |
|||||||||||
|
Biopsying clinician |
|
|
|
|
|||||||||||
|
General practitioner |
404 (72%) |
96 (17%) |
69 (12%) |
569 |
|||||||||||
|
Dermatologist |
125 (55%) |
26 (11%) |
86 (35%) |
237 |
|||||||||||
|
Surgeon |
265 (91%) |
14 (5%) |
14 (5%) |
293 |
|||||||||||
|
Undetermined |
39 (75%) |
7 (13%) |
6 (12%) |
52 |
|||||||||||
|
|
|||||||||||||||
|
Incisional biopsy (seven patients) and curettage (12 patients) not shown. ◆ |
|||||||||||||||
Box 2 – Diagnostic biopsy techniques, by year and tumour thickness
|
|
2005 |
2010 |
2015 |
Total |
|||||||||||
|
|
|||||||||||||||
|
Type of biopsy |
400 |
400 |
400 |
1200 |
|||||||||||
|
Excisional |
314 (78%) |
274 (69%) |
245 (62%) |
833 |
|||||||||||
|
Partial (punch, shave, incisional, curettage) |
80 (20%) |
111 (28%) |
146 (36%) |
337 |
|||||||||||
|
Diagnostic biopsy unavailable |
6 (2%) |
15 (3%) |
9 (2%) |
30 |
|||||||||||
|
Thin tumour (< 1.0 mm) * |
197 |
187 |
189 |
573 |
|||||||||||
|
Excisional biopsy |
146 (73%) |
121 (61%) |
109 (55%) |
376 |
|||||||||||
|
All partial biopsy types |
51 (26%) |
70 (35%) |
85 (42%) |
206 |
|||||||||||
|
Shave biopsy |
29 (15%) |
39 (20%) |
62 (31%) |
130 |
|||||||||||
|
Punch biopsy |
22 (11%) |
27 (14%) |
18 (9%) |
67 |
|||||||||||
|
Incisional or curettage |
0 |
4 (2%) |
5 (3%) |
9 |
|||||||||||
|
Intermediate tumour (1.0–4.0 mm) † |
98 |
97 |
98 |
293 |
|||||||||||
|
Excisional biopsy |
82 (82%) |
72 (72%) |
62 (62%) |
216 |
|||||||||||
|
All partial biopsy type |
16 (16%) |
26 (26%) |
38 (38%) |
80 |
|||||||||||
|
Shave biopsy |
6 (6%) |
12 (12%) |
14 (14%) |
32 |
|||||||||||
|
Punch biopsy |
10 (10%) |
13 (13%) |
22 (22%) |
45 |
|||||||||||
|
Incisional or curettage |
0 |
1 (1%) |
2 (2%) |
3 |
|||||||||||
|
Thick tumour (> 4.0 mm) † |
97 |
94 |
94 |
285 |
|||||||||||
|
Excisional biopsy |
86 (86%) |
81 (81%) |
74 (74%) |
241 |
|||||||||||
|
All partial biopsy types |
13 (13%) |
14 (14%) |
24 (24%) |
51 |
|||||||||||
|
Shave biopsy |
2 (2%) |
7 (7%) |
4 (4%) |
13 |
|||||||||||
|
Punch biopsy |
9 (9%) |
6 (6%) |
16 (16%) |
31 |
|||||||||||
|
Incisional or curettage |
2 (2%) |
1 (1%) |
4 (4%) |
7 |
|||||||||||
|
|
|||||||||||||||
|
* Denominator: 200 patients in each year. † Denominator: 100 patients in each year. ◆ |
|||||||||||||||
Box 4 – Diagnostic biopsy techniques employed, by biopsying clinician type
|
Practitioner type |
2005 |
2010 |
2015 |
Total* |
|||||||||||
|
|
|||||||||||||||
|
General practitioner (family physician) |
179 |
175 |
215 |
369 [38%] |
|||||||||||
|
Excisional biopsy |
139 (78%) |
126 (72%) |
139 (65%) |
204 [31%] |
|||||||||||
|
Shave biopsy |
10 (6%) |
19 (11%) |
40 (19%) |
69 [39%] |
|||||||||||
|
Punch biopsy |
30 (17%) |
30 (17%) |
36 (17%) |
96 [67%] |
|||||||||||
|
Dermatologist |
76 |
86 |
75 |
237 [24%] |
|||||||||||
|
Excisional biopsy |
47 (62%) |
46 (53%) |
32 (43%) |
125 [19%] |
|||||||||||
|
Shave biopsy |
22 (29%) |
30 (35%) |
34 (45%) |
86 [49%] |
|||||||||||
|
Punch biopsy |
7 (9%) |
10 (12%) |
9 (12%) |
26 [18%] |
|||||||||||
|
Surgeon |
116 |
104 |
73 |
293 [30%] |
|||||||||||
|
Excisional biopsy |
110 (95%) |
93 (89%) |
62 (85%) |
265 [40%] |
|||||||||||
|
Shave biopsy |
4 (3%) |
7 (7%) |
3 (4%) |
14 [8%] |
|||||||||||
|
Punch biopsy |
2 (2%) |
4 (4%) |
8 (11%) |
14 [10%] |
|||||||||||
|
Undetermined |
27 |
28 |
27 |
82 [8%] |
|||||||||||
|
Excisional biopsy |
24 (89%) |
24 (86%) |
21 (78%) |
69 [10%] |
|||||||||||
|
Shave biopsy |
1 (4%) |
2 (7%) |
3 (11%) |
6 [3%] |
|||||||||||
|
Punch biopsy |
2 (7%) |
2 (7%) |
3 (11%) |
7 [5%] |
|||||||||||
|
|
|||||||||||||||
|
With proportions of all skin biopsies or biopsy techniques attributable to practitioner type. ◆ |
|||||||||||||||
Box 5 – Number of shave biopsies associated with base transection and T‐subsequent upstaging, by tumour thickness category
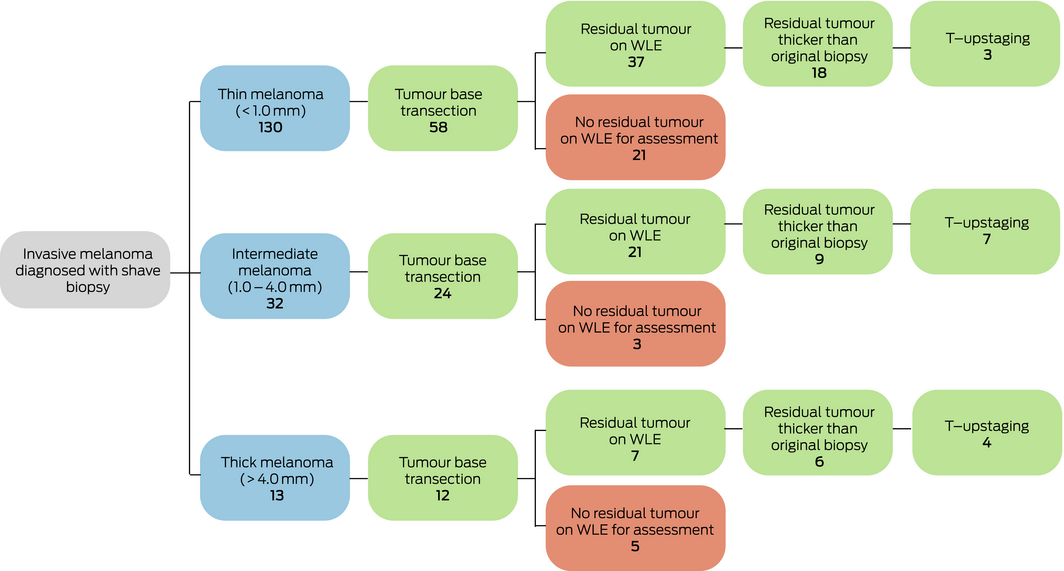
WLE = wide local excision. ◆
Box 6 – Underestimation of Breslow thickness, by partial biopsy technique: mean values (with standard deviations) in millimetres
|
|
All melanomas |
Thin melanomas |
Intermediate melanomas |
Thick melanomas |
|||||||||||
|
|
|||||||||||||||
|
All techniques (punch, shave, curette, incisional) |
0.80 (3.99) |
0.09 (0.19) |
0.50 (0.80) |
4.48 (9.91) |
|||||||||||
|
All punch biopsies |
1.50 (5.91) |
0.16 (0.23) |
0.64 (0.81) |
5.86 (12.1) |
|||||||||||
|
All shave biopsies |
0.25 (1.27) |
0.06 (0.16) |
0.35 (0.80) |
2.15 (4.53) |
|||||||||||
|
Shave biopsies with tumour base transection |
0.46 (1.82) |
0.07 (0.18) |
0.48 (0.93) |
2.36 (4.72) |
|||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 16 January 2019, accepted 22 March 2019





Abstract
Objective: To assess changes in the choice of skin biopsy technique for assessing invasive melanoma in Victoria, and to examine the impact of partial biopsy technique on the accuracy of tumour microstaging.
Design: Retrospective cross‐sectional review of Victorian Cancer Registry data on invasive melanoma histologically diagnosed in Victoria during 2005, 2010, and 2015.
Setting, participants: 400 patients randomly selected from each of the three years, stratified by final tumour thickness: 200 patients with thin melanoma (< 1.0 mm), 100 each with intermediate (1.0–4.0 mm) and thick melanoma (> 4.0 mm).
Main outcome measures: Partial and excisional biopsies, as proportions of all skin biopsies; rates of tumour base transection and T‐upstaging, and mean tumour thickness underestimation following partial biopsy.
Results: 833 excisional and 337 partial diagnostic biopsies were undertaken. The proportion of partial biopsies increased from 20% of patients in 2005 to 36% in 2015 (P < 0.001); the proportion of shave biopsies increased from 9% in 2005 to 20% in 2015 (P < 0.001), with increasing rates among dermatologists and general practitioners. Ninety‐four of 175 shave biopsies (54%) transected the tumour base; wide local excision subsequently identified residual melanoma in 65 of these cases (69%). Twenty‐one tumours diagnosed by shave biopsy (12%) were T‐upstaged. With base‐transected shave biopsies, tumour thickness was underestimated by a mean 2.36 mm for thick, 0.48 mm for intermediate, and 0.07 mm for thin melanomas.
Conclusion: Partial biopsy, particularly shave biopsy, was increasingly used for diagnosing invasive melanoma between 2005 and 2015. Shave biopsy has a high rate of base transection, reducing the accuracy of tumour staging, which is crucial for planning appropriate therapy, including definitive surgery and adjuvant therapy.