The known: In December 2017, the National Cervical Screening Program shifted from cytology‐based screening to primary human papillomavirus (HPV)‐based screening.
The new: The proportion of HPV16/18‐positive women with high grade cytologic abnormalities was higher than for women positive for other HPV types, which supports the differential management of these women. As predicted by clinical trial and modelling data, rates of referral to colposcopy increased after the switch to primary HPV screening.
The implications: These early observations suggest the renewed program is performing as expected during the first screening round, but timely program monitoring is critical for ensuring community confidence in the new policy.
Improved understanding of human papillomavirus (HPV) epidemiology and advances in molecular detection of HPV have led to major innovations in cervical cancer prevention strategies, including highly effective prophylactic HPV vaccines and a shift from cytology‐ to HPV‐based primary cervical screening.1,2 Consequently, the Australian National Cervical Screening Program underwent a major paradigm shift in December 2017, switching from biennial cytological Pap testing of asymptomatic women aged 18–69 years to 5‐yearly primary HPV testing of women aged 25–74 years.3 This policy (the Renewal) was informed by a comprehensive evidence review, a health economics assessment, and mathematical modelling, all undertaken in the context of the highly successful HPV vaccination program introduced in Australia in 2007.4,5
In the renewed program, asymptomatic women are initially invited to undergo primary HPV testing at age 25, with an exit test at age 70–74 years. Testing involves partial genotyping (for HPV16 and 18) followed by reflex liquid‐based cytology (LBC) if any oncogenic HPV is detected.3 Women are subsequently managed according to their risk of significant cervical abnormality during the following 5 years (low, intermediate, or higher risk) as indicated by the screening test result (Box 1). Co‐testing (HPV testing and LBC) is recommended for all women (regardless of age) being followed up after treatment of a high grade abnormality or who are classed as being at risk of cervical cancer because of symptoms or clinical signs.3
In the long term, primary HPV testing is expected to have substantial advantages over cytology‐based screening, including major cost savings and reduced incidence and mortality of cervical cancer.4,6 However, significant fluctuations in health outcomes and operational aspects of the program (rates of follow‐up and colposcopy referral) are also expected.6,7 While national data will be critical for tracking performance, timely local monitoring can provide important early insights into key indicators of the program while it is still in its infancy.
In this article, we report key cross‐sectional results for more than 195 000 primary screening and non‐screening tests submitted to a large pathology laboratory during the first 6 months of the Renewal program. We report oncogenic HPV‐positivity rates by reason for test referral. We also estimated HPV‐positivity rates in screening tests for women in the age band recommended for primary HPV screening, as well as rates of recommendations for 12‐month follow‐up and colposcopy.
Methods
We undertook a retrospective cross‐sectional study of all cervical samples submitted for HPV testing by medical practitioners to a large community‐based general pathology laboratory in metropolitan Sydney between 1 December 2017 and 31 May 2018. The laboratory receives referrals from general practitioners, reproductive health clinics, and specialist gynaecologists in Sydney, from regional cities and rural areas of New South Wales, and from South Australia. Each sample was collected by a clinician in a vial of PreservCyt transport medium (Hologic) suitable for both HPV testing and LBC, in accordance with Renewal requirements.8
Upon receipt by the laboratory, specimens were classified according to the management guidelines:3 testing of specimens from women with clinical symptoms or signs or from women who were being followed up after an earlier abnormality were classified as “non‐screening”; all other tests were classified as “primary screening”. These categories were based on the patient history in our laboratory information system, the National Cancer Screening Register, and state Pap test registries, and on information provided by the clinician, including specific symptoms or signs that may have motivated the request for co‐testing. Classification was double‐checked when a test result was validated.
HPV testing was performed with the clinically validated diagnostic platform, the Roche cobas 6800 (Roche Diagnostics), approved by the Therapeutic Goods Administration for the renewed program.8 The assay detects 14 oncogenic HPV types (16/18/31/33/35/39/45/51/52/56/58/59/66/68) and reports results for HPV16, HPV18, and “other”. As an internal quality control measure, an “invalid” result was reported in instances of test inhibition or poor cellularity (failure to detect the internal cellular control, β‐globin). Specimens from HPV‐positive women were assessed by reflex LBC; the slides were examined by a cytologist and referred to a gynaecological cytopathologist if any abnormality was seen.3,8 When co‐testing of a specimen was required, LBC was performed after HPV testing. LBC results were reported using Australian Modified Bethesda System terminology.3,8
We estimated the prevalence (with 95% confidence intervals [CIs] estimated by the binomial exact method) of any oncogenic HPV, of HPV16 or 18 (HPV16/18), and of other oncogenic HPV without detection of HPV16/18 (non‐16/18), stratified by reason for test referral (primary screening, non‐screening). Rates were expressed as the proportion of valid tests (internal cellular control detected) with positive results. For non‐screening tests, HPV prevalence was estimated for two Medicare‐designated referral groups: follow‐up for prior low grade abnormality, and co‐test (reasons for co‐testing: clinical signs or symptoms, test of cure after treatment of high grade squamous intraepithelial lesion [HSIL], prior adenocarcinoma in situ, or indication unknown). For primary screening samples from women aged 25–74 years, we estimated HPV prevalence by 5‐year age group. For HPV‐positive specimens, we estimated rates of cervical low grade abnormality (low grade squamous intraepithelial lesion [LSIL] or possible LSIL) and high grade abnormality (HSIL, possible HSIL, adenocarcinoma in situ, or cancer) as indicated by reflex LBC; we also estimated the proportion of women classified as being at low, intermediate or higher risk of cervical abnormality (Box 1).3 Statistical analyses were performed in Stata 15.1 (StataCorp).
Ethics approval
The study was approved by the Royal Women's Hospital Human Research and Ethics Committee (audit/quality assurance no. 18/46).
Results
During the first 6 months of Renewal, 195 606 samples were received by the pathology laboratory — 164 976 (84.3%) from NSW, 30 630 (15.7%) from SA — of which 157 700 (80.6%) were for primary screening and 37 906 (19.4%) for non‐screening tests (including 12 703 [33.5%] for co‐tests following symptoms or abnormal clinical signs). A total of 221 tests (0.11%; 95% CI, 0.10–0.13%) were invalid. Oncogenic HPV was detected in 12 699 valid primary screening tests (8.1%; 95% CI, 7.9–8.2%); HPV16/18 was detected in 3453 (2.2%; 95% CI, 2.1–2.3%) and non‐16/18 types in 9246 screening tests (5.9%; 95% CI, 5.8–6.0%). Oncogenic HPV was detected in 7900 non‐screening tests (20.9%; 95% CI, 20.5–21.3%); HPV16/18 in 1606 (4.2%; 95% CI, 4.0–4.5%) and non‐16/18 types in 6294 tests (16.6%; 95% CI, 16.3–17.0%) (Box 2).
Age‐specific prevalence of oncogenic human papillomaviruses
Of 157 700 primary screening tests, 860 (0.6%) were for women outside the recommended age for screening; this included 725 for women under 25 (353 [48.6%] during the first 2 months of Renewal, and 108 [14.9%] during month 6). A total of 157 primary screening tests (0.10%; 95% CI, 0.08–0.12) were invalid. The prevalence of HPV16/18 was highest among women aged 30–34 years (2.8%; 95% CI, 2.6–3.0%) and was only slightly lower in older age groups. In contrast, the prevalence of non‐16/18 oncogenic HPV types was highest in women aged 25–29 years (16.2%; 95% CI, 15.7–16.8%) and declined sharply with age (Box 3; Supporting Information, table 1).
Age‐specific prevalence of cervical abnormality detected by reflex cytology
Of 12 479 HPV‐positive screening test specimens from women in the recommended age range for screening (Supporting Information, table 1), 92 (0.7%; 95% CI, 0.6–0.9%) were unsatisfactory on reflex LBC. Of 3397 HPV16/18‐positive specimens, 1236 (36.4%; 95% CI, 34.8–38.1%) had cytological cervical abnormalities: 715 (21.1%; 95% CI, 19.7–22.5%) were low grade, 521 (15.3%; 95% CI, 14.2–16.6%) were high grade abnormalities. The prevalence of low grade abnormality was highest in women aged 25–29 years (28.8%; 95% CI, 24.0–34.1%) or 45–49 years (26.0%; 95% CI, 21.9–30.6%). The prevalence of high grade abnormality was highest in women aged 25–29 (21.2%; 95% CI, 17.0–26.2%) or 30–34 years (21.7%; 95% CI, 18.5–25.3%). Of 8990 non‐HPV16/18‐positive specimens, 3165 (35.2%; 95% CI, 34.2–36.2%) had a cytologic cervical abnormality: 2600 (28.9%; 95% CI, 28.0–29.9%) were low grade, 565 (6.3%; 95% CI, 5.8–6.8%) were high grade abnormality. The prevalence of low grade abnormality was highest in women aged 25–29 years (33.2%; 95% CI, 31.4–35.0%), while the prevalence of high‐grade abnormality was higher across a broader age range (25–44 years) (Box 4, Box 5; Supporting Information, table 2).
Risk classification and management recommendations
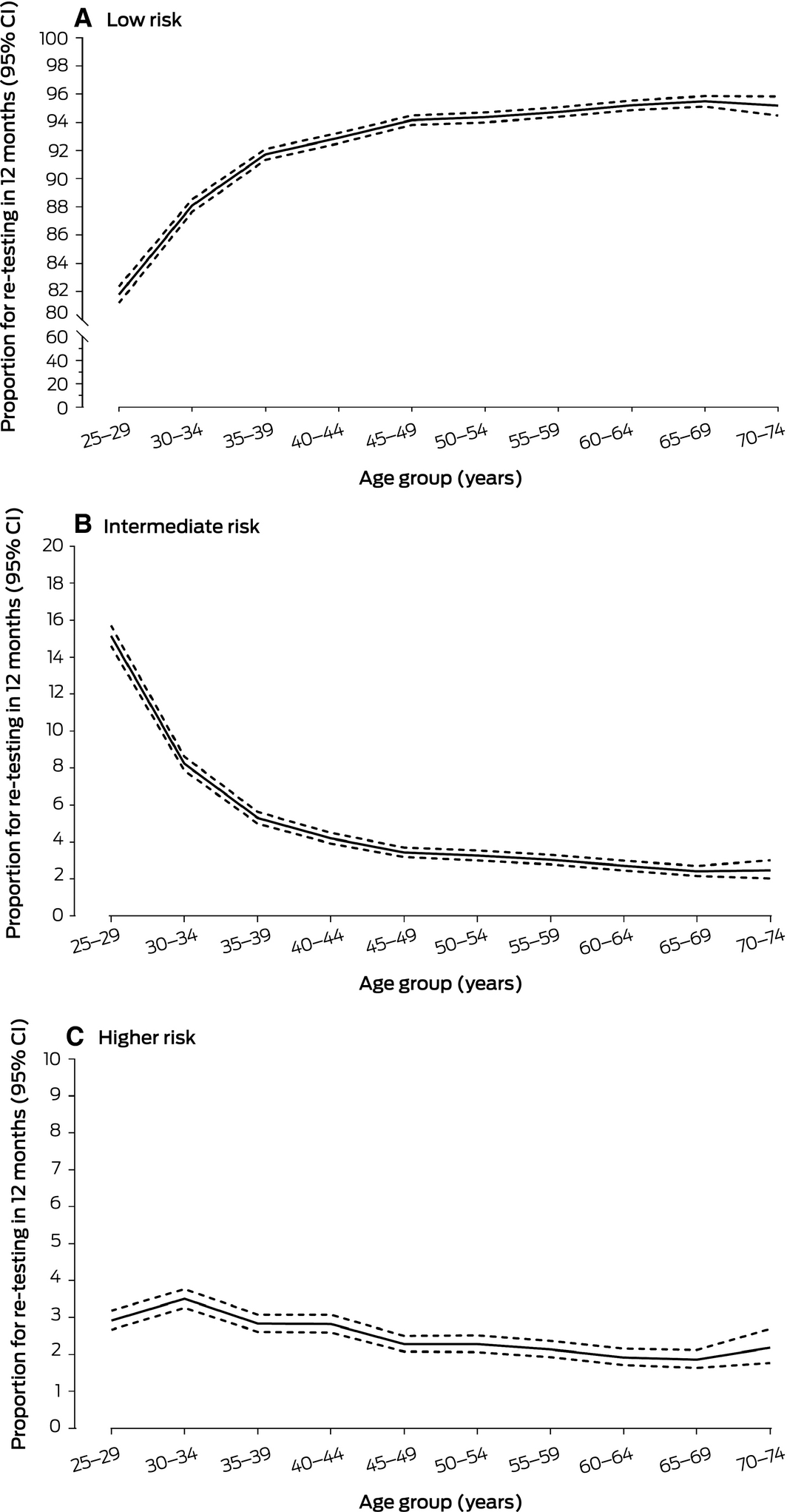
Follow‐up HPV testing after 12 months (intermediate risk) was recommended in 8425 cases (5.4%; 95% CI, 5.3–5.5%), and direct colposcopy referral (higher risk) in 4006 (2.6%; 95% CI, 2.5–2.7%). The proportion for whom 12‐month follow‐up testing was recommended was greatest for women aged 25–29 years (15.2%; 95% CI, 14.6–15.7%); the colposcopy referral rate was greatest for women aged 30–34 years (3.5%; 95% CI, 3.3–3.8%) (Box 6, Box 7; Supporting Information, table 3).
Discussion
More than 195 000 specimens were submitted to a large community‐based pathology laboratory during the first 6 months of the renewed National Cervical Screening Program; in 8% of primary screening tests and 21% of non‐screening tests the samples were positive for oncogenic HPV. The prevalence of oncogenic HPV types other than HPV16/18 in screening specimens from women aged 25–74 years (the age range recommended for screening) declined sharply with age, whereas that of HPV16/18 was low and similar across age groups. This pattern is consistent with reports on the impact of HPV vaccination.9,10 Just over one‐third of oncogenic HPV‐positive samples also exhibited cytologic abnormalities, but the proportion of HPV16/18‐positive specimens with high grade abnormalities was greater than for those positive for other HPV types, supporting the higher risk classification of women with HPV16/18‐positive specimens.
The Renewal program distinguishes between HPV specimens submitted for primary screening and those submitted for other indications (non‐screening), requiring laboratories to classify all tests accordingly for Medicare billing purposes and for patient management.3,8 Women with non‐screening tests are regarded as being at higher risk than other women because of their symptoms or signs or a prior cervical abnormality. This was reflected by the higher oncogenic HPV prevalence in non‐screening than screening samples. It was highest (35%) in women being followed up for low grade cytologic changes; these are usually not treated, as most are caused by self‐limiting infections that will spontaneously resolve.11 Prevalence was lower among those followed up after therapy for high grade changes (18%), reflecting successful treatment of most of these women. HPV prevalence in women with symptoms or signs (15%) was also higher than in the screening population. The recommendation to co‐test women with symptoms and signs was based on the acknowledged limitations of HPV testing for detecting infection in the presence of excess blood, which can be present in patients with invasive carcinoma.12 However, application of this category differs between clinicians, with anecdotal reports of overuse, leading the National Cervical Screening Program to further specify the definitions of relevant signs and symptoms.13
A key finding was that the rate of referral to colposcopy based on HPV primary screening sample results for women of recommended screening age (2.6%) was considerably higher than that based on historical primary cytology screening results from our laboratory (0.8%; unpublished data). The higher rate is broadly consistent with clinical trial data and predictions from modelling.7,14 It had been anticipated that more high grade abnormalities and cancer would be detected during the first round of the renewed program than previously because HPV testing is more sensitive than cytology‐based screening. Colposcopy referral rates are expected to decline in subsequent screening rounds, when it will predominantly be incident disease that is detected.6
While the sensitivity for detecting high grade abnormalities is greater for HPV testing than for cytology, its specificity is considerably lower,1,15 so that referring all HPV‐positive women to colposcopy would result in many unnecessary procedures. Partial HPV genotyping improves test specificity by allowing direct referral of women positive for the most oncogenic HPV types.11,16 In our study, high grade cytologic abnormalities were indeed more common in women positive for HPV16/18. Similarly, 12‐month surveillance of women positive for other oncogenic HPV types but who had no or a low grade cytologic abnormalities is intended to mitigate the risk associated with HPV infections that are likely to be transient.1,3 Rates of recommended surveillance varied greatly with age, and were higher for younger women. The results of follow‐up testing will provide important information about the subsequent risk of HSIL and its relationship with age.
The age‐specific patterns of HPV prevalence we found are consistent with recent Renewal data from a large laboratory in Victoria,9 and the similar prevalence of HPV16/18 in both populations reflects the documented impact of HPV vaccination.17 Uptake of HPV vaccination in Australia was more rapid and extensive than in other countries, profoundly reducing the population‐level prevalence of the targeted HPV types and of clinical endpoints in all vaccination‐eligible groups.10,17
Limitations
The National Cancer Screening Register was not fully functional when the renewed program commenced on 1 December 2017. An important consequence was that complete screening histories were not available for several months, so that some non‐screening tests may have been misclassified as screening tests. We largely overcame this problem by checking our own laboratory records and those of the state Pap test registries. A further limitation of our report is the absence of follow‐up histological data, which are often not available until months after the screening report has been issued. Other important questions, such as the presence of HSIL without cytologic abnormality and the positive predictive values of the various levels in the cytology report, will be discussed in a separate article. As we analysed an extract of de‐identified data, we were unable to identify and remove any repeat tests, but this problem is unlikely to be significant, especially for screening tests, given the short time frame of the study; a woman can have only one primary screening test every 5 years. Finally, the results reflect those of a single laboratory and may not be generalisable nationally. There are currently few comparative data, but the patterns of oncogenic HPV prevalence in our sample and in the Victorian study9 may well be generalisable across Australia.
The strength of our report is that it reflects real world experience of the renewed program, including a very large volume of tests with a single platform (Roche 6800).18 HPV testing in the Australian program can be undertaken with any approved assay.8,19 The impact of this decision on the ongoing consistency and reproducibility of the program has not been fully resolved, and strict quality assurance measures have been implemented to monitor inconsistencies.8 The laboratory in our report has rigorous quality assurance measures and a specialised cervical screening unit, ensuring internal validity of its results.
Conclusion
The switch from cytology‐ to primary HPV‐based screening in Australia will ensure cervical screening is evidence‐based and best practice. While the predicted long term benefits are substantial, timely monitoring of the transitional phase is critical for ensuring the program performs as expected and community confidence in the policy is maintained.
Box 1 – Risk‐based management recommendations in the renewed National Cervical Screening Program3
|
Result of primary screening human papillomavirus (HPV) test |
Risk of cervical abnormality within 5 years |
Recommendation |
|||||||||||||
|
|
|||||||||||||||
|
Negative for oncogenic HPV types |
Low risk |
Re‐test in 5 years |
|||||||||||||
|
Other oncogenic HPV detected (non‐16/18)* and reflex LBC result is negative or low grade abnormality† |
Intermediate risk |
12‐month follow‐up |
|||||||||||||
|
HPV16 or 18 detected;‡ or other oncogenic HPV detected (non‐16/18)* and reflex LBC result is high grade abnormality§ |
Higher risk |
Refer for colposcopy |
|||||||||||||
|
Invalid HPV results or unsatisfactory LBC samples |
Unsatisfactory |
Repeat screening test |
|||||||||||||
|
|
|||||||||||||||
|
LBC = liquid‐based cytology. * Positive for one or more of HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, or 68, without detection of HPV16 or 18. † Low grade squamous intraepithelial lesion (LSIL) or possible LSIL. ‡ Regardless of reflex LBC result. § High grade squamous intraepithelial lesion (HSIL), possible HSIL, adenocarcinoma in situ, or cancer. ◆ |
|||||||||||||||
Box 2 – Reasons for testing of 195 606 samples received for human papillomavirus (HPV) testing, December 2017 – May 2018, and overall proportions of positive HPV results in valid tests
|
Reason for HPV test† |
All tests |
Valid tests* |
Positive test result |
||||||||||||
|
Any oncogenic HPV |
HPV16/18 |
Other oncogenic HPV only (non‐16/18) |
|||||||||||||
|
Number |
Proportion |
Number |
Proportion |
Number |
Proportion |
||||||||||
|
|
|||||||||||||||
|
Primary screening |
157 700 (80.6%) |
157 542 |
12 699 |
8.1% |
3453 |
2.2% |
9246 |
5.9% |
|||||||
|
Non‐screening |
37 906 |
37 843 |
7900 |
20.9% |
1606 |
4.2% |
6294 |
16.6% |
|||||||
|
Follow‐up for prior LSIL |
6118 |
6096 |
2106 |
34.6% |
380 |
6.2% |
1726 |
28.3% |
|||||||
|
Co‐test for prior AIS |
49 |
49 |
4 |
8% |
1 |
2% |
3 |
6% |
|||||||
|
Co‐test for prior HSIL |
9682 |
9672 |
1708 |
17.7% |
532 |
5.5% |
1176 |
12.2% |
|||||||
|
Co‐test for symptoms/signs |
12 703 |
12 685 |
1954 |
15.4% |
324 |
2.6% |
1630 |
12.9% |
|||||||
|
Co‐test, indication unknown |
9354 |
9341 |
2128 |
22.8% |
369 |
4.0% |
1759 |
18.8% |
|||||||
|
|
|||||||||||||||
|
AIS = adenocarcinoma in situ; CI = confidence interval; HSIL = high‐grade squamous intraepithelial lesion; LSIL = low‐grade squamous intraepithelial lesion. * β‐globin‐positive. † Tests for women with symptoms or signs, or from women who were being followed up for a prior abnormality were classified as non‐screening tests; all other tests were classified as primary screening tests.3 ◆ |
|||||||||||||||
Box 3 – Age‐specific prevalence of oncogenic human papillomavirus (HPV) in 156 683 valid primary screening tests from women aged 25–74 years, December 2017 – May 2018
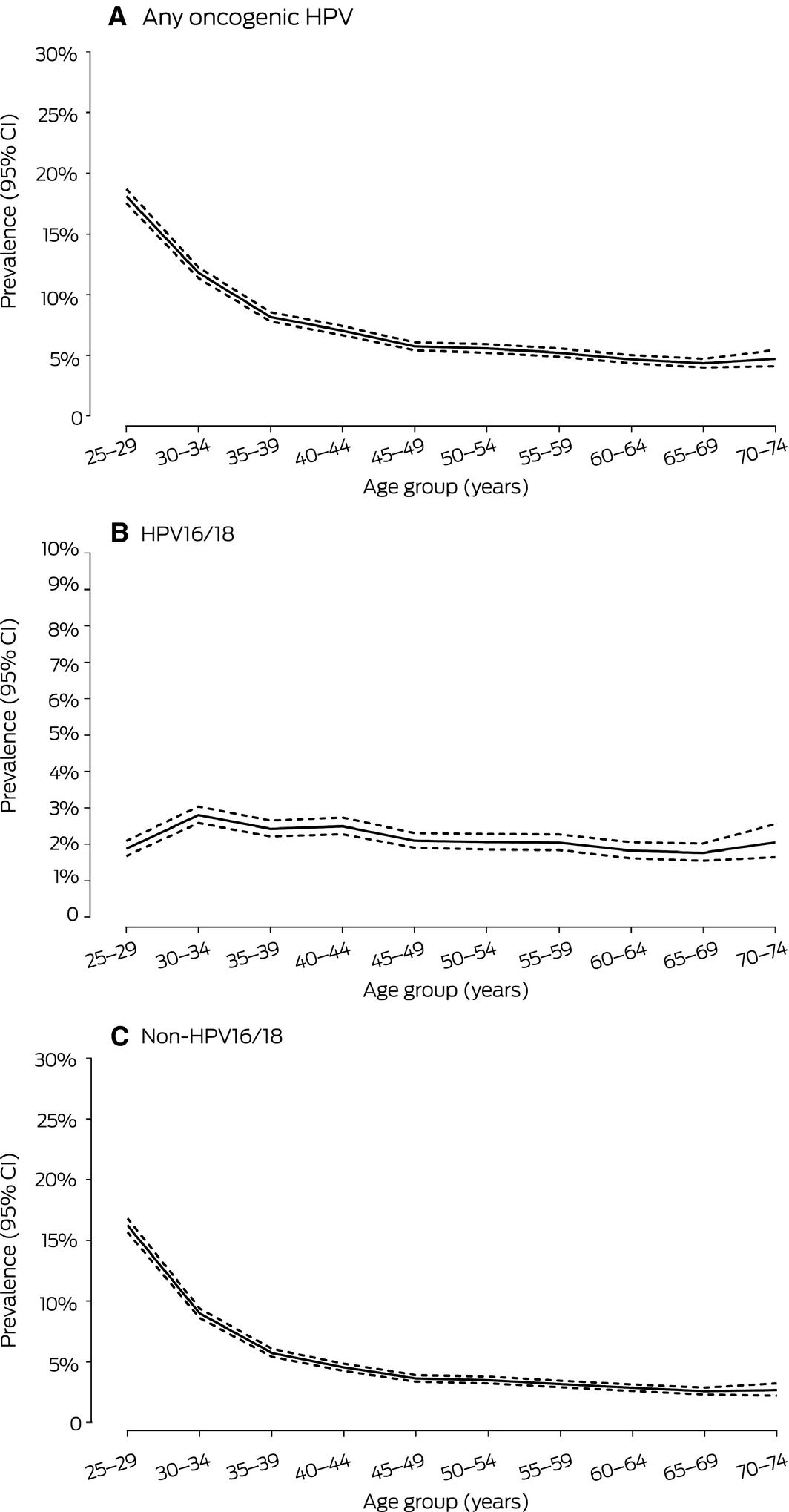
CI = confidence interval. ◆
Box 4 – Age‐specific prevalence of cervical abnormality detected by reflex cytology in 12 387 oncogenic human papillomavirus (HPV)‐positive primary screening test specimens from women aged 25–74 years, December 2017 – May 2018
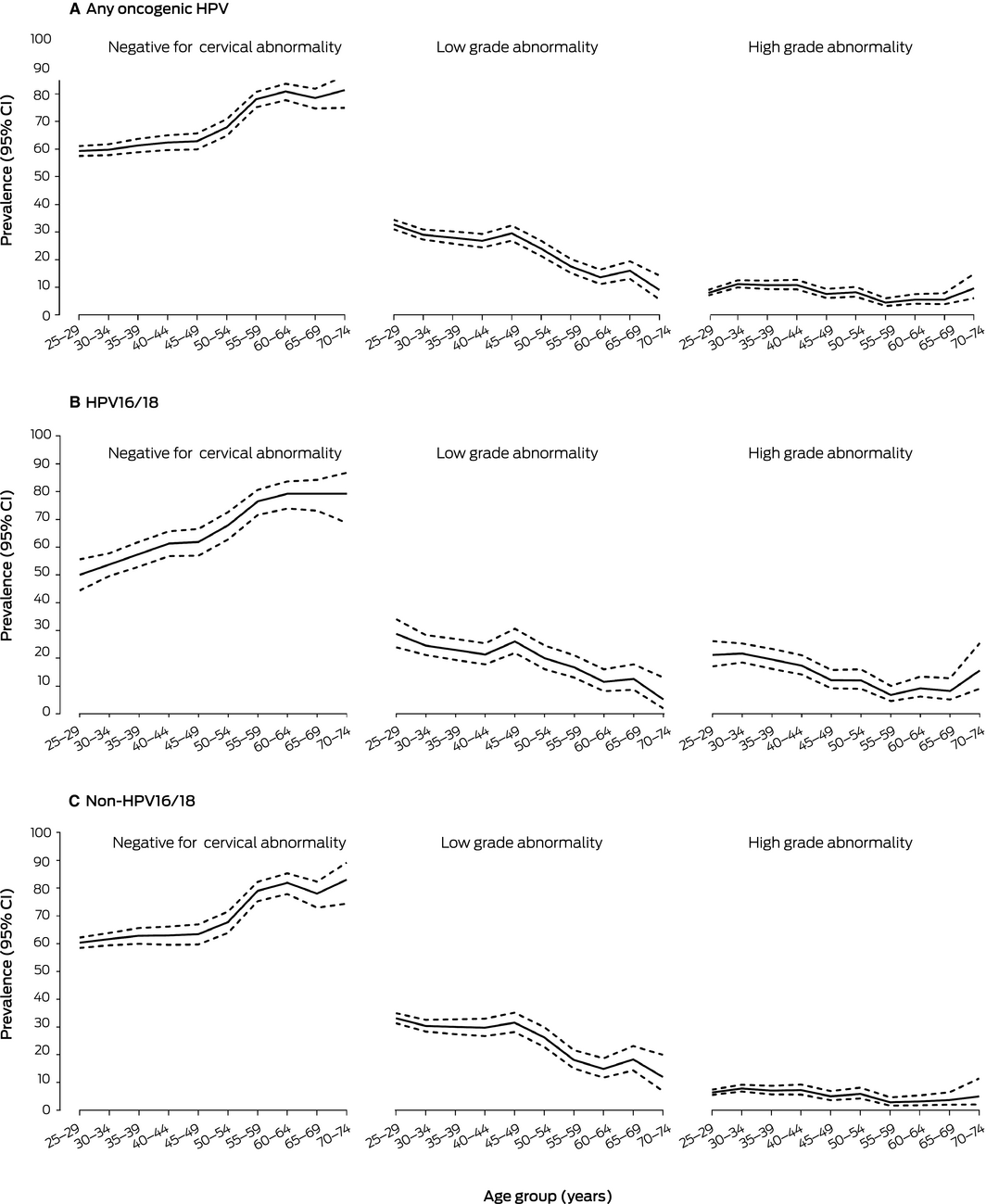
CI = confidence interval. ◆
Box 5 – Results of reflex liquid‐based cytology for 12 387 oncogenic human papillomavirus (HPV)‐positive primary screening test specimens from women aged 25–74 years, December 2017 – May 2018
|
Highest grade of cervical abnormality |
Positive screening test result |
||||||||||||||
|
Any oncogenic HPV |
HPV16/18 |
Other oncogenic HPV only (non‐16/18) |
|||||||||||||
|
Number |
Proportion |
Number |
Proportion |
Number |
Proportion |
||||||||||
|
|
|||||||||||||||
|
Total number of tests |
12 387 |
|
3397 |
|
8990 |
|
|||||||||
|
Negative for cervical abnormality |
7986 |
64.5% |
2161 |
63.6% |
5825 |
64.8% |
|||||||||
|
Any cervical abnormality |
4401 |
35.5% |
1236 |
36.4% |
3165 |
35.2% |
|||||||||
|
Low grade abnormality* |
3315 |
26.8% |
715 |
21.1% |
2600 |
28.9% |
|||||||||
|
High grade abnormality† |
1086 |
8.8% |
521 |
15.3% |
565 |
6.3% |
|||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Low grade squamous intraepithelial lesion (LSIL) or possible LSIL. † High grade squamous intraepithelial lesion (HSIL; 469), possible HSIL (590), adenocarcinoma in situ (12), squamous cell cancer (one), atypical endocervical cells of undermined significance (six); possible high grade glandular lesion (four), or mixed adenocarcinoma in situ and HSIL (four). ◆ |
|||||||||||||||
Box 6 – Risk classification and management recommendations following 156 840 primary human papillomavirus (HPV) screening tests from women aged 25–74 years, December 2017 – May 2018
|
Risk classification* |
Recommendation |
Number of tests |
Proportion of screening tests |
||||||||||||
|
|
|||||||||||||||
|
Low risk |
Re‐test in 5 years |
144 204 |
91.9% |
||||||||||||
|
Intermediate risk |
12‐month follow‐up |
8425 |
5.4% |
||||||||||||
|
Higher risk† |
Refer for colposcopy |
4006 |
2.6% |
||||||||||||
|
Unsatisfactory‡ |
Repeat screening test |
205 |
0.13% |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Definitions: see Box 1. † The HPV test for five specimens were invalid, but high grade changes were detected by reflex liquid‐based cytology. ‡ For 142 specimens, HPV tests were invalid and the reflex liquid‐based cytology results were unsatisfactory; 53 were positive for other oncogenic HPV types but were unsatisfactory on cytology; ten had invalid HPV tests but had no or low grade changes on reflex liquid‐based cytology. ◆ |
|||||||||||||||
Received 28 November 2018, accepted 5 March 2019





Abstract
Objectives: To report human papillomavirus (HPV) testing patterns and rates of oncogenic HPV‐positivity for specimens submitted during the first 6 months after the National Cervical Screening Program switched from cytology‐ to primary HPV‐based screening.
Design, participants: Retrospective cross‐sectional review of 195 606 specimens submitted for HPV testing, 1 December 2017 – 31 May 2018.
Setting: Large community‐based general pathology laboratory in metropolitan Sydney.
Main outcome measures: Prevalence of oncogenic HPV types (all, HPV16/18, non‐HPV16/18) by reason for HPV test (primary screening, non‐screening); for oncogenic HPV‐positive women in the age band recommended for primary HPV screening (25–74 years), prevalence of cytologic abnormality and rates of 12‐month follow‐up and colposcopy recommendations.
Results: 195 606 samples were received: 157 700 (80.6%) for primary screening, 37 906 (19.4%) for non‐screening tests. Oncogenic HPV was detected in 8.1% of screening tests (95% CI, 7.9–8.2%) and 20.9% of non‐screening tests (95% CI, 20.5–21.3%); 35.5% (95% CI, 34.7–36.4%) of women of recommended screening age with positive oncogenic HPV screening test results also had a cytologic abnormality. The proportion of HPV16/18‐positive samples with high grade abnormality was 15.3% (95% CI, 14.2–16.6%); for samples positive for other oncogenic HPV types, the proportion was 6.3% (95% CI, 5.8–6.8%). Repeat HPV testing after 12 months was recommended for 5.4% (95% CI, 5.3–5.5%) and direct colposcopy for 2.6% (95% CI, 2.5–2.7%) of screened women aged 25–74 years.
Conclusions: High grade cytologic abnormalities were more common in women positive for HPV16/18, supporting their higher risk classification. Colposcopy referral rates were higher than during primary cytology‐based testing, as predicted by clinical trial and modelling data. The prevalence of HPV was much higher in non‐screening than in primary screening samples. Our findings indicate the renewed program is performing as expected during the initial HPV screening round.