The known: Acute kidney injury (AKI) is linked with short and long term morbidity and mortality, including progression to chronic kidney disease (CKD). Unlike CKD, the prevalence of AKI in Indigenous Australians has not been documented.
The new: The age distribution of AKI events among Indigenous Australians in the Kimberley was shifted to younger age groups than in national data, and AKI was more frequently associated with skin infections. One‐third of events were detected on or before the date of hospital admission.
The implications: Systems‐based approaches to identifying and managing patients with AKI, and preventing infections could improve health outcomes for Indigenous Australians.
The incidence of acute kidney injury (AKI), which is strongly associated with considerable early and long term morbidity and mortality, is rising around the world.1,2,3 AKI is defined as an abrupt decline in kidney function indicated by an acute increase in serum creatinine level, with or without reduced urine output.4 Detecting AKI early facilitates supportive management, including fluid resuscitation, maintenance of normal blood pressure, and avoidance of nephrotoxins.5 Conversely, undetected or unmanaged AKI may lead to adverse outcomes, including chronic kidney disease (CKD).6 The link between AKI and the development of CKD, including in people with apparently complete recovery of renal function,6,7,8 is increasingly recognised.7,9,10
The only review of the epidemiology of AKI in Australia was published by the Australian Institute of Health and Welfare (AIHW) in 2015.2 The review, based on International Classification of Diseases, tenth revision (ICD‐10) data in the National Hospital Morbidity Database, reported that the hospitalisation rate was increasing, particularly for patients aged 60 years or more who also had cardiovascular or respiratory system disease.2 Differences in the rates of hospitalisation and mortality related to age, remoteness of residence, socio‐economic status, and Indigenous status were noted, but specific patterns were not described.2
About 1.7 million Australians have CKD,11 and the total costs attributable to CKD in 2012 were estimated to be $4.1 billion, including $2.5 billion in direct health care costs.12 The greater burden of CKD among Australian Aboriginals and Torres Strait Islanders (Indigenous Australians) than among other Australians is well documented; the incidence of end‐stage kidney disease is 4.9 times as high among Indigenous men and 8.0 times as high among Indigenous women as among non‐Indigenous Australians,13 and the incidence of CKD increases with remoteness.13,14 Further, the mean age of Indigenous patients commencing renal replacement therapy (RRT) is lower.15 However, the prevalence and aetiology of AKI in Indigenous Australians has not been documented.
The CKD summit of the International Society of Nephrology (July 2016) concluded that strong systems‐based approaches to preventing and detecting AKI early are needed, and that risk factors at the regional level should be identified.16 Accordingly, we investigated the epidemiology of AKI events in a region with a large Indigenous Australian population, with the aim of identifying opportunities for reducing the high incidence of CKD among Aboriginal and Torres Strait Islander Australians.
Methods
We employed a retrospective population‐based study design. Cases of AKI during the period 1 June 2009 – 30 May 2016 were identified in data extracted from Kimberley electronic medical record systems (MMEx, ISA Healthcare; Communicare, HealthConnex). The analysis included data for all individuals documented as being Aboriginal or Torres Strait Islander Australians, aged 15 years or more at the time of renal function testing, not having end‐stage kidney disease, and for whom at least two serum creatinine level values were recorded within 7 days of one another (Box 1).
Setting
The Kimberley region is very remote and has a relatively large Indigenous Australian population (2016: 14 299 people, 42% of the total population).17 Acute dialysis is not available in the region, and there is no intensive care unit. The nearest nephrology services are in Perth (more than 2000 km away) and Darwin (over 800 km away). CKD rates are high, and the regional capacity for satellite dialysis (120 patients) is insufficient; at the time of writing, 25 patients are in Perth on waiting lists for dialysis in the Kimberley.
Acute kidney injury events
AKI events were identified as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria:5 an absolute increase in serum creatinine level within 48 hours of at least 26.5 μmol/L, or a relative increase within 7 days of at least 50%. AKI events were confirmed by manual review of electronic medical records. To estimate baseline CKD stage, the estimated glomerular filtration rate (eGFR) value prior to the AKI event was calculated from creatinine values with the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula.18 AKI events were excluded from our analysis if they were dated after the onset of end‐stage kidney disease (defined by three consecutive eGFR values below 15 mL/min/1.73m2 over at least 6 months) or the commencement of renal replacement therapy (including haemodialysis, peritoneal dialysis, or renal transplantation).
Data collection and analysis
Principal and additional diagnoses were obtained from discharge summaries, or from primary care medical records when there was no hospital admission or the discharge summary was not available. If no diagnosis was defined as the principal diagnosis, the first diagnosis was deemed the principal diagnosis. Diagnoses were categorised by ICD‐10 codes and cross‐checked by two authors. Discharge summaries were assessed for recording of prior CKD and of AKI as a principal or additional diagnosis.
Creatinine values and test dates were imported into Excel 2010 (Microsoft) for analysis. Descriptive statistical analysis was completed in Stata 14 (StataCorp). Population data from the 2016 census were used for calculating age‐specific AKI rates for the entire audit, as the Kimberley Indigenous population was stable during this period.17 Age groups were aggregated to match those in the AIHW AKI review;2 95% confidence intervals (CIs) for crude rates were calculated as the standard error × 1.96.
Ethics approval
This project received ethics approval from the Western Australian Aboriginal Health Ethics Committee (reference, 701) and the WA Country Health Service Human Research Ethics Committee (reference, 2016/07), and was supported by the Kimberley Aboriginal Health Planning Forum Research Subcommittee.
Results
Data for 324 AKI events in 260 people were identified (Box 2). Two hundred and fifteen individuals (83%) had single events, 32 (12%) had two, nine (3%) had three, three (1%) had four, and one (0.3%) had six events. The overall AKI rate was 323 per 100 000 population (95% CI, 281–367 per 100 000 population; for people aged 15 years or more: 479 per 100 000 population; 95% CI, 427–532 per 100 000 population). The median age of patients at the time of their AKI was 51.8 years (interquartile range [IQR], 43.9–61.0 years; range, 18.1–90.6 years), with approximately equal numbers of events in male and female patients (Box 3). Ninety‐two of the 324 events (28%) were in people aged 15–44 years (Box 3), but the rates increased with age (events per 100 000 population: 15–24 years, 46 [95% CI, 30–62]; 34–44 years, 460 [95% CI, 409–512]; 75–84 years, 1617 [95% CI, 1521–1714]) (Box 4).
Hospitalisations and discharge summary content
Two hundred and seventy nine of 294 events for which the hospitalisation status was available (95%) were associated with a hospital admission; for 54 of the 279 events (19%), the patients were transferred to a tertiary centre, including four who underwent acute dialysis. The date of admission was available for 230 events; 78 events (34%) were detected on or before the day of admission (Box 3).
Of the 224 events for which discharge summaries were available, AKI was coded as the principal or first diagnosis for 18 events (8%) and as a secondary diagnosis for 43 events (19%); a history of CKD was recorded for 105 events (47%). Eighty‐three of 224 events (37%) were in patients without CKD diagnoses in their discharge summaries and who had eGFR values exceeding 60 mL/min/1.73m2 prior to the AKI.
Principal and additional diagnoses
A diagnosis was available for 289 of the 324 events (89%). For 88 of these 289 events (30%) a single diagnosis was recorded or the principal diagnosis was documented; for 201 events the first diagnosis listed was accepted as the principal diagnosis and others as additional diagnoses. The three most frequent ICD‐10 diagnostic categories were diseases of the respiratory tract (60 of 324 events, 18%), the skin and subcutaneous tissues (41 events, 13%), and the urogenital tract (33 events, 10%); the most frequent specific diagnoses were pneumonia (39 events, 12%), infections of the skin and subcutaneous tissue (33 events, 10%), and urinary tract infections (25 events, 7.7%) (Box 5). Overall, 149 of 289 principal diagnoses (52%) were infectious in nature, and for a further 19 events (7%) an infective diagnosis was listed as an additional diagnosis (data not shown).
Discussion
We report the first population‐based study in which AKI events were identified in the medical records of an Australian cohort by applying the KDIGO criteria, and the first study to examine the epidemiology of AKI in a cohort of Indigenous Australians. The age distribution of events and the pattern of associated diagnoses differed from those for the broader Australian population as recorded in the National Hospital Morbidity Database.2
Although differences in methodology prevent direct comparison of age‐specific AKI rates, the age distribution of events among Indigenous Australians in the Kimberley differed from that for Australians in general; in particular, more than one‐quarter of events were in people under 45 years of age, compared with 8%2 for all Australians. The higher AKI rates in younger age groups mirror the higher incidence of end‐stage kidney disease among younger Indigenous Australians.19 The AKI rates for Kimberley Indigenous people over 65 years of age were, in contrast, lower than the corresponding national rates, possibly reflecting a healthy survivor effect, a cohort effect, an increased likelihood of random error because of the small numbers of Kimberley Indigenous people in these age groups, or less frequent renal function testing of Indigenous people who are over 65.
We found that skin infections were among the most frequent principal diagnoses for Indigenous people in the Kimberley with an AKI event; in the national data, in contrast, the top ten diagnostic categories of principal diagnoses for hospitalisations in which AKI was an additional diagnosis did not include skin conditions.2 A large burden of skin disease in Indigenous Australians has been reported, but mainly in children;20,21,22 we found that the negative impact of skin infections extends into adulthood. Indigenous communities with housing problems and overcrowding as environmental risk factors are more likely to report skin conditions as health problems,23 and streptococcal skin infections are associated with acute post‐infectious glomerulonephritis and CKD.24 The association between infections and AKI in our study reinforces the importance of addressing the underlying environmental and economic determinants of health, and of reducing barriers to identifying and treating infections in a timely manner.25 This will require coordinating the activities of environmental health organisations, primary care bodies, and hospitals.
AKI can be identified early and managed with the aim of reducing the likelihood of complications. At the 2016 CKD summit of the International Society of Nephrology, it was recommended that systems for identifying patients with CKD be developed, that individuals with risk factors for AKI be identified, that kidney function be monitored in high risk clinical scenarios or following relevant exposures, and that medical record alerts be provided to medical practitioners in the event of an AKI.16 When implementing such strategies, it is important to note that one‐third of events in our study were detected before or on the day of hospital admission, indicating that AKI can be prevented and identified in community as well as in hospital settings.
As risk factors for AKI (including proteinuria and pre‐existing CKD26) are common among Indigenous Australians,2 awareness among doctors of the substantial proportion of people at high risk for AKI should be promoted, as well as of the fact that many (and perhaps most) events begin outside hospitals, so that prevention is at least as important as in‐hospital management. Our study indicates the importance of primordial prevention; that is, attending to overcrowding and other social determinants of health. Additionally, primary prevention of infections by evidence‐based, community‐driven primary health programs could prevent a range of associated sequelae, and reduce the need for repeated courses of antibiotics in an era of increasing antibiotic resistance.27 These are important areas for future research, evaluation, and continuous quality improvement.
Limitations
Our study was limited by its retrospective design and the need for a baseline creatinine value from the 7 days preceding the AKI event. We will also have missed events detected during the recovery phase after an initial creatinine rise. The authors of a recent multinational study using a similar KDIGO‐based methodology noted the same limitation, and modified the criteria to allow measures of baseline renal function that were up to 12 months old, but they commented that this probably led to some instances of progressive CKD being misclassified as AKI.28 This problem would be particularly relevant in our study population, in which CKD is common. Further, data on urine output, a complementary component of the KDIGO definition of AKI (output less than 0.5 mL/kg/h for at least 6 hours5), were not available for our analysis.
As a result of these limitations, we probably underestimated the number of AKI events. Our results would also be biased toward events diagnosed in hospitals with regular creatinine monitoring. Our data consequently do not provide an accurate estimate of the incidence of AKI, but indicate demographic and aetiological patterns that could be useful for informing clinical practice.
The age distribution and pattern of diagnoses we described is limited to a particular setting, a relatively remote area with a large Indigenous population in the tropical north of Western Australia. Analysis of data at the regional level can provide useful information for developing policy, but our findings may not be generalisable to other regions. While AKI is a recognised risk factor for CKD, data on the rate of progressive renal dysfunction and mortality are not available. Fewer than one‐third of the discharge summaries we reviewed included AKI as a principal or additional diagnosis, suggesting that AKI is undercoded by hospital physicians. This has implications for estimating the burden of disease and for communicating to primary care providers that the AKI event is a health risk factor that requires follow‐up. If events are not recognised, opportunities for reducing their impact may be missed.
Conclusion
Our study illustrates the utility of the KDIGO criteria for investigating AKI at the population level and the benefits of combining the analysis of pathology, hospitalisation, and primary care data sets at the regional level. Further, we highlight a recognised risk factor for CKD and the opportunity to reduce its impact by reviewing and improving aspects of clinical care for Indigenous Australians in community and hospital settings in the Kimberley and other remote areas of Australia.
Box 1 – Identifying cases of acute kidney injury in Indigenous Australians in the Kimberley region, 1 June 2009 – 30 May 2016
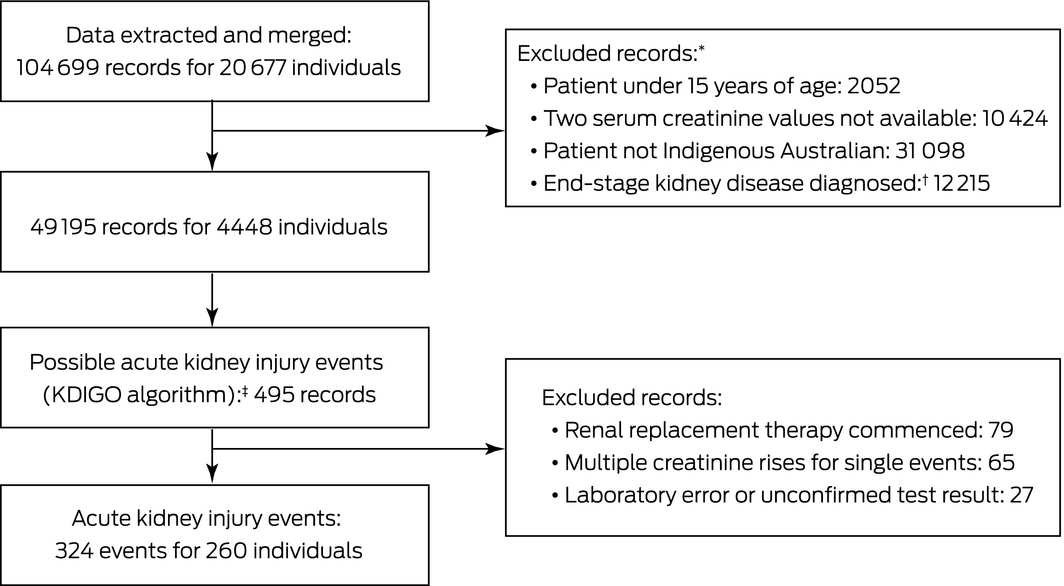
KDIGO = Kidney Disease: Improving Global Outcomes.5 * Exclusion for multiple reasons possible. † Defined by three consecutive estimated glomerular filtration rate values under 15 mL/min/1.73 m2 over 6 months. ‡ Defined by an increase in serum creatinine level to at least 1.5 times baseline within 7 days, or by at least 26.5 μmol/L within 48 hours. ◆
Box 2 – Distribution of 324 identified acute kidney injury events in Indigenous Australians in the Kimberley region, June 2009 – May 2016, by year*
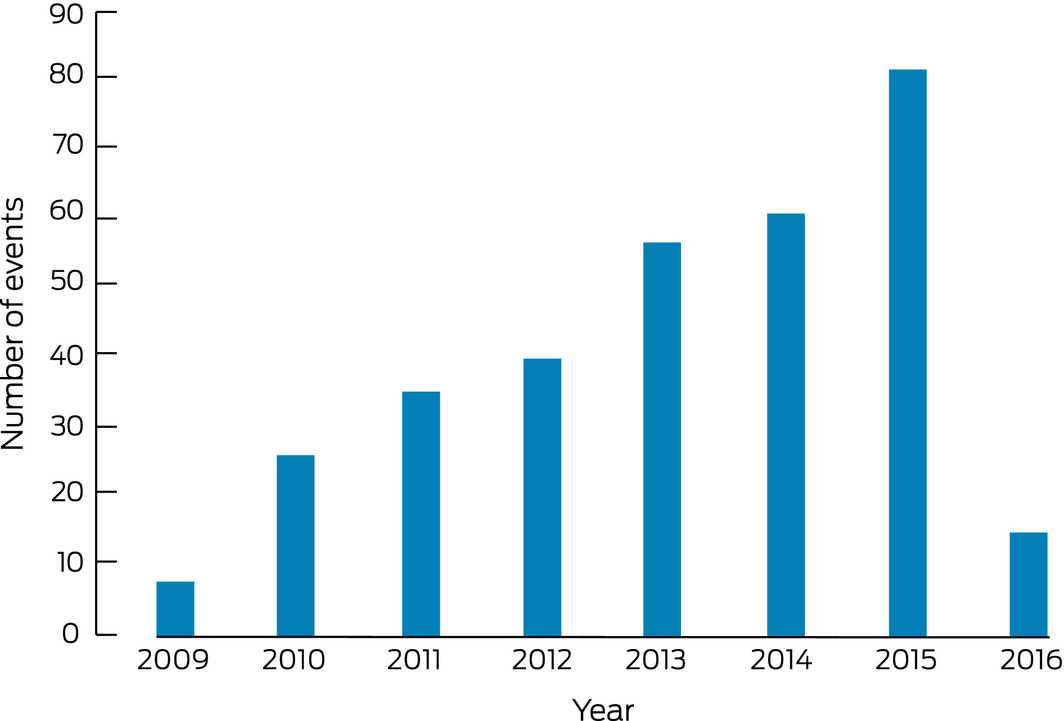
* Data for 2009 and 2016 are incomplete (only portions of each year were included in study period). ◆
Box 3 – Characteristics of Indigenous Australians in the Kimberley region with an acute kidney injury, June 2009 – May 2016
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Total number of acute kidney injury (AKI) events |
324 |
||||||||||||||
|
Age (proportion of Kimberley Indigenous population17) |
|||||||||||||||
|
15–24 years (17%) |
8 (2%) |
||||||||||||||
|
25–34 years (16%) |
26 (8.0%) |
||||||||||||||
|
35–44 years (13%) |
58 (18%) |
||||||||||||||
|
45–54 years (10%) |
101 (31%) |
||||||||||||||
|
55–64 years (6.9%) |
72 (22%) |
||||||||||||||
|
65–74 years (2.9%) |
33 (10%) |
||||||||||||||
|
75–84 years (1.1%) |
18 (5.6%) |
||||||||||||||
|
85 or more years (0.4%) |
8 (2%) |
||||||||||||||
|
Sex |
|||||||||||||||
|
Male |
176 (54%) |
||||||||||||||
|
Female |
148 (46%) |
||||||||||||||
|
AKI stage (based on change in serum creatinine levels)* |
|||||||||||||||
|
1 |
235 (72%) |
||||||||||||||
|
2 |
20 (6.2%) |
||||||||||||||
|
3 |
69 (21%) |
||||||||||||||
|
Hospitalisation status unknown |
30 |
||||||||||||||
|
Hospitalisation status known |
294 |
||||||||||||||
|
None |
15 (5.1%) |
||||||||||||||
|
Regional hospital only |
223 (76%) |
||||||||||||||
|
Regional hospital, with transfer to tertiary hospital |
54 (18%) |
||||||||||||||
|
Tertiary hospital only |
2 (0.7%) |
||||||||||||||
|
Hospitalised patients: day of AKI detection unknown |
49 |
||||||||||||||
|
Hospitalised patients: day of AKI detection known |
230 |
||||||||||||||
|
Day of admission or earlier |
78 (34%) |
||||||||||||||
|
Day 2 of admission |
57 (24%) |
||||||||||||||
|
Day 3 of admission |
46 (20%) |
||||||||||||||
|
Day 4 of admission or later |
49 (21%) |
||||||||||||||
|
Estimated glomerular filtration rate prior to AKI event (mL/min/1.73 m2) |
|||||||||||||||
|
≥ 60 |
171 (53%) |
||||||||||||||
|
30–59 |
79 (24%) |
||||||||||||||
|
15–29 |
48 (15%) |
||||||||||||||
|
< 15 |
26 (8.0%) |
||||||||||||||
|
|
|||||||||||||||
|
* Kidney Disease: Improving Global Outcomes (KDIGO) definitions:5 stage 1, 1.5–1.9 times baseline or increased by at least 26.5 μmol/L; stage 2, 2.0–2.9 times baseline; stage 3, 3.0 times baseline or at least 353.6 μmol/L. ◆ |
|||||||||||||||
Box 4 – Age group‐specific annual rates of acute kidney injury events among Indigenous Australians in the Kimberley region (KDIGO criteria5), 2009–2016,* compared with rates for all Australians, 2012–13 (National Hospital Morbidity Database2)
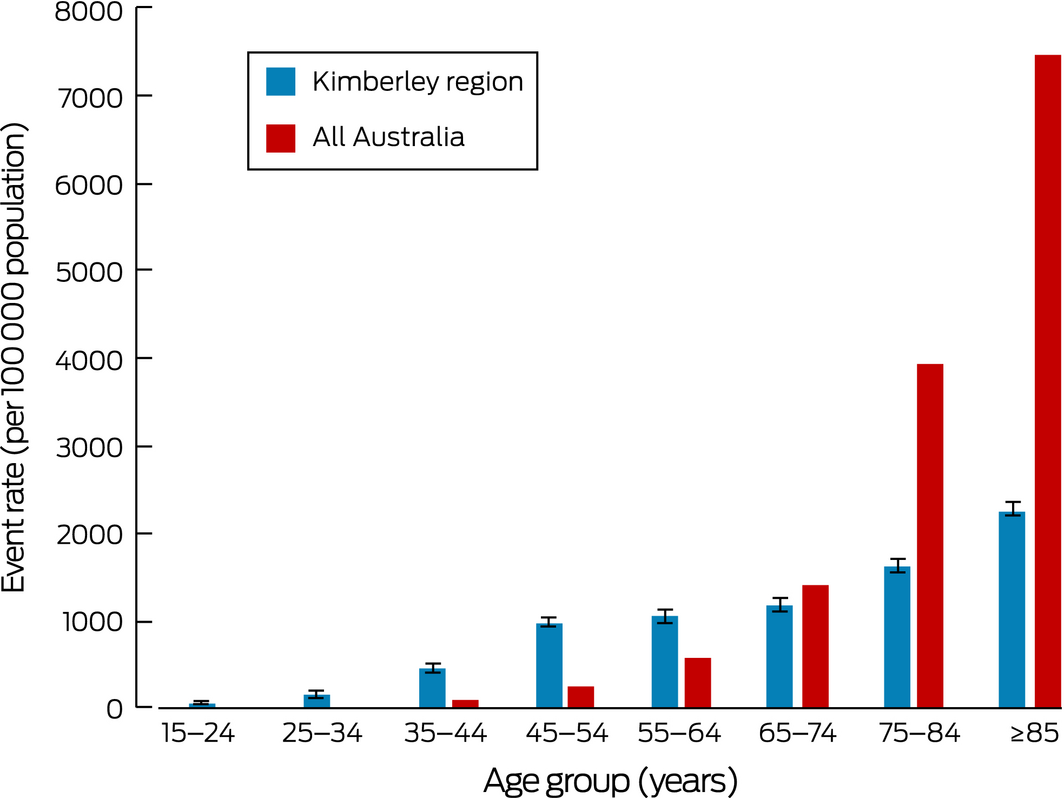
* Population estimates for Kimberley people based on 2016 national census data;17 error bars denote 95% confidence intervals (standard error × 1.96). ◆
Box 5 – Acute kidney injury events in Indigenous Australians in the Kimberley region, June 2009 – May 2016, by category of principal diagnosis (International Statistical Classification of Diseases, 10th revision) and by principal diagnosis
|
Diagnosis |
Acute kidney injury events |
||||||||||||||
|
|
|||||||||||||||
|
Total number of acute kidney injury events |
324 |
||||||||||||||
|
Principal diagnosis, by ICD‐10 category |
|||||||||||||||
|
Diseases of the respiratory system |
60 (18%) |
||||||||||||||
|
Diseases of the skin and subcutaneous tissue |
41 (13%) |
||||||||||||||
|
Diseases of the genitourinary system (other than acute kidney injury) |
33 (10%) |
||||||||||||||
|
Diseases of the circulatory system |
28 (8.6%) |
||||||||||||||
|
Diseases of the digestive system |
22 (6.8%) |
||||||||||||||
|
Diseases of the genitourinary system (includes acute kidney injury) |
21 (6.5%) |
||||||||||||||
|
Diseases of the musculoskeletal system and connective tissue |
16 (4.9%) |
||||||||||||||
|
Certain infectious and parasitic diseases |
15 (4.6%) |
||||||||||||||
|
Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified |
13 (4.0%) |
||||||||||||||
|
Endocrine, nutritional and metabolic diseases |
11 (3.4%) |
||||||||||||||
|
Diseases of the nervous system |
7 (2%) |
||||||||||||||
|
Other* |
22 (6.8%) |
||||||||||||||
|
No documentation available |
35 (11%) |
||||||||||||||
|
Principal diagnosis, specific |
|||||||||||||||
|
Acute kidney injury/acute renal failure |
21 (6.5%) |
||||||||||||||
|
Pneumonia |
39 (12%) |
||||||||||||||
|
Infections of the skin and subcutaneous tissues |
33 (10%) |
||||||||||||||
|
Urinary tract infection, including pyelonephritis |
25 (7.7%) |
||||||||||||||
|
Acute pulmonary oedema |
8 (2%) |
||||||||||||||
|
Sepsis, source unspecified |
8 (2%) |
||||||||||||||
|
Respiratory tract infection, other |
7 (2%) |
||||||||||||||
|
Infection, other‡ |
7 (2%) |
||||||||||||||
|
Diabetic foot ulcer/infection |
6 (2%) |
||||||||||||||
|
Heart failure |
6 (2%) |
||||||||||||||
|
Acute coronary syndrome |
6 (2%) |
||||||||||||||
|
Dehydration or hypotension |
5 (2%) |
||||||||||||||
|
Pancreatitis |
5 (2%) |
||||||||||||||
|
Liver failure |
5 (2%) |
||||||||||||||
|
Gastroenteritis |
4 (1%) |
||||||||||||||
|
Hyponatraemia |
4 (1%) |
||||||||||||||
|
Osteomyelitis |
3 (1%) |
||||||||||||||
|
Septic arthritis |
3 (1%) |
||||||||||||||
|
Cholecystitis |
3 (1%) |
||||||||||||||
|
Cerebrovascular event |
3 (1%) |
||||||||||||||
|
Other |
88 (27%) |
||||||||||||||
|
No documentation available |
35 (11%) |
||||||||||||||
|
|
|||||||||||||||
|
* Neoplasms, injury and poisoning, mental and behavioural disorders, diseases of the blood and blood‐forming organs and certain disorders involving the immune mechanism, external causes of morbidity and mortality, pregnancy, childbirth and the puerperium, and diseases of the ear and mastoid process. † Acute coronary syndrome, acute tubular necrosis, diabetic nephropathy, nephrotoxic medication, obstructive uropathy, small bowel obstruction, urinary tract infection. ‡ Melioidosis, bronchiectasis, infective endocarditis, scabies, varicella zoster infection. ◆ |
|||||||||||||||
Received 8 July 2018, accepted 30 October 2018
- Joseph V Mohan1,2
- David N Atkinson2
- Johan B Rosman3
- Emma K Griffiths2,4
- 1 The University of Western Australia, Perth, WA
- 2 Rural Clinical School of Western Australia, University of Western Australia, Broome, WA
- 3 Curtin University, Perth, WA
- 4 Kimberley Aboriginal Medical Services, Broome, WA
We thank Julia Marley (Kimberley Research, University of Western Australia) for critically reviewing our manuscript.
No relevant disclosures.
- 1. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371: 58–66.
- 2. Australian Institute of Health and Welfare. Acute kidney injury in Australia: a first national snapshot (Cat. No. PHE 190). Canberra: AIHW, 2015.
- 3. Cerda J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol 2008; 4: 138–153.
- 4. Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013; 84: 457–467.
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138.
- 6. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int 2012; 81: 442–448.
- 7. Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82: 516–524.
- 8. Parr SK, Matheny ME, Abdel‐Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2017; 93: 460–469.
- 9. Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011; 171: 226–233.
- 10. Ponte B, Felipe C, Muriel A, et al. Long‐term functional evolution after an acute kidney injury: a 10‐year study. Nephrol Dial Transplant 2008; 23: 3859–3866.
- 11. Australian Institute of Health and Welfare. Chronic kidney disease. Dec 2017. https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/chronic-kidney-disease/overview (viewed Jan 2019).
- 12. Wyld MLR, Lee CMY, Zhuo X, et al. Cost to government and society of chronic kidney disease stage 1–5: a national cohort study. Intern Med J 2015; 45: 741–747.
- 13. Australian Institute of Health and Welfare. Chronic kidney disease in Aboriginal and Torres Strait Islander people, 2011 (Cat. No. PHE 151). Canberra: AIHW, 2011.
- 14. Hoy WE, Mott SA, McDonald SP. An expanded nationwide view of chronic kidney disease in Aboriginal Australians. Nephrology (Carlton) 2016; 21: 916–922.
- 15. Marley JV, Dent HK, Wearne M, et al. Haemodialysis outcomes of Aboriginal and Torres Strait Islander patients of remote Kimberley region origin. Med J Aust 2010; 193: 516–520. https://www.mja.com.au/journal/2010/193/9/haemodialysis-outcomes-aboriginal-and-torres-strait-islander-patients-remote
- 16. Levin A, Tonelli M, Bonventre J, et al; ISN Global Kidney Health Summit participants. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917.
- 17. Australian Bureau of Statistics. 2016 Census QuickStats: Kimberley. Updated 13 Dec 2018. http://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/51001?opendocument (viewed Jan 2019).
- 18. Levey AS, Stevens LA, Schmid CH, et al; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612.
- 19. Australia and New Zealand Dialysis and Transplant Registry. End stage kidney disease among Indigenous Peoples of Australia and New Zealand. In: 38th annual ANZDATA report (2015). Adelaide: ANZDATA Registry, 2016; pp. 12/1–12/23. http://www.anzdata.org.au/anzdata/AnzdataReport/38thReport/c12_anzdata_indigenous_v3.0_201600128_web.pdf (viewed Jan 2018).
- 20. Hendrickx D, Bowen AC, Marsh JA, et al. Ascertaining infectious disease burden through primary care clinic attendance among young Aboriginal children living in four remote communities in Western Australia. PLoS One 2018; 13: e0203684.
- 21. Abdalla T, Hendrickx D, Fathima P, et al. Hospital admissions for skin infections among Western Australian children and adolescents from 1996 to 2012. PLoS One 2017; 12: e0188803.
- 22. Aung PTZ, Cuningham W, Hwang K, et al. Scabies and risk of skin sores in remote Australian Aboriginal communities: a self‐controlled case series study. PLoS Negl Trop Dis 2018; 12: e0006668.
- 23. Melody SM, Bennett E, Clifford HD, et al. A cross‐sectional survey of environmental health in remote Aboriginal communities in Western Australia. Int J Environ Health Res 2016; 26: 525–535.
- 24. Hoy WE, White AV, Dowling A, et al. Post‐streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int 2012; 81: 1026–1032.
- 25. White A, Wong W, Sureshkumur P, Singh G. The burden of kidney disease in indigenous children of Australia and New Zealand, epidemiology, antecedent factors and progression to chronic kidney disease. J Paediatr Child Health 2010; 46: 504–509.
- 26. Rewa O, Bagshaw SM. Acute kidney injury: epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10: 193–207.
- 27. May PJ, Bowen AC, Carapetis JR. The inequitable burden of group A streptococcal diseases in Indigenous Australians. Med J Aust 2016; 205: 201–203. https://www.mja.com.au/journal/2016/205/5/inequitable-burden-group-streptococcal-diseases-indigenous-australians
- 28. Mehta RL, Burdmann EA, Cerdá J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross‐sectional study. Lancet 2016; 387: 2017–2025.





Abstract
Objective: To describe the frequencies of acute kidney injury (AKI) and of associated diagnoses in Indigenous people in a remote Western Australian region.
Design: Retrospective population‐based study of AKI events confirmed by changes in serum creatinine levels.
Setting, participants: Aboriginal and Torres Strait Islander residents of the Kimberley region of Western Australia, aged 15 years or more and without end‐stage kidney disease, for whom AKI between 1 June 2009 and 30 May 2016 was confirmed by an acute rise in serum creatinine levels.
Main outcome measures: Age‐specific AKI rates; principal and other diagnoses.
Results: 324 AKI events in 260 individuals were recorded; the median age of patients was 51.8 years (IQR, 43.9–61.0 years), and 176 events (54%) were in men. The overall AKI rate was 323 events (95% CI, 281–367) per 100 000 population; 92 events (28%) were in people aged 15–44 years. 52% of principal diagnoses were infectious in nature, including pneumonia (12% of events), infections of the skin and subcutaneous tissue (10%), and urinary tract infections (7.7%). 80 events (34%) were detected on or before the date of admission; fewer than one‐third of discharge summaries (61 events, 28%) listed AKI as a primary or other diagnosis.
Conclusion: The age distribution of AKI events among Indigenous Australians in the Kimberley was skewed to younger groups than in the national data on AKI. Infectious conditions were common in patients, underscoring the significance of environmental determinants of health. Primary care services can play an important role in preventing community‐acquired AKI; applying pathology‐based criteria could improve the detection of AKI.