Studies in the United States have raised concerns about the increasing concomitant prescribing of opioids and benzodiazepines and the incidence of associated serious harms, including respiratory depression, hospitalisation, and death.1,2,3 As a result, and following their own 2016 review, the Food and Drug Administration decided that opioid and benzodiazepine medications in the US must include boxed warnings about possible serious side‐effects.4 Whether concomitant prescribing has also increased in Australia is unknown.
We conducted a retrospective population‐based cohort study of the concomitant dispensing of opioids and benzodiazepines in Australia between 1 July 2012 and 30 June 2017 (ethics approval: University of South Australia; reference, P099/10). We analysed a 10% sample of de‐identified Pharmaceutical Benefits Scheme (PBS) claims data provided to us by the Australian Department of Human Services to estimate the proportions of people aged 18 years or more who were dispensed PBS‐listed opioids or benzodiazepines. Concurrent dispensing was defined as a benzodiazepine being dispensed to an individual within a week of their being dispensed an opioid. The duration of an episode of concurrent dispensing was calculated from the date of dispensing and the prescription duration. To examine changes over time, rate ratios (RRs) were estimated in Poisson regression models that accounted for correlation of monthly rates.
An estimated 631 940 people (2.8% of the Australian population) were dispensed an opioid during July 2012, and 722 380 (2.9%) in June 2017; the mean annual increase was 1.0% (RR, 1.01; 95% confidence interval [CI], 1.00–1.02). An estimated 389 610 people (1.7%) were dispensed a benzodiazepine in July 2012, 360 200 (1.5%) during June 2017; the mean annual decrease was 2.9% (RR, 0.97; 95% CI, 0.97–0.98).
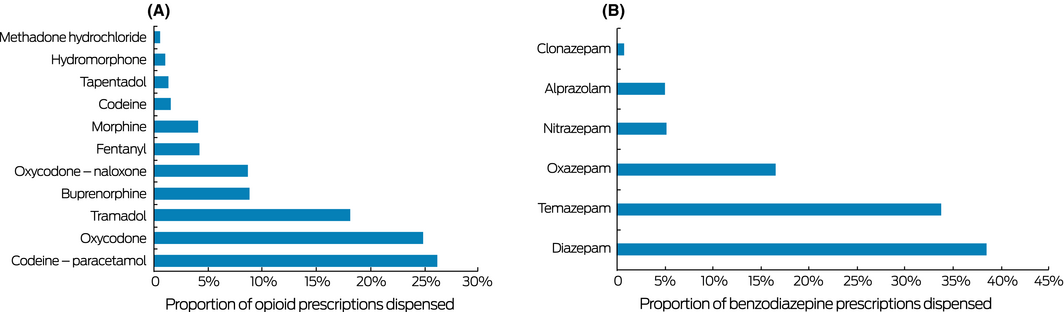
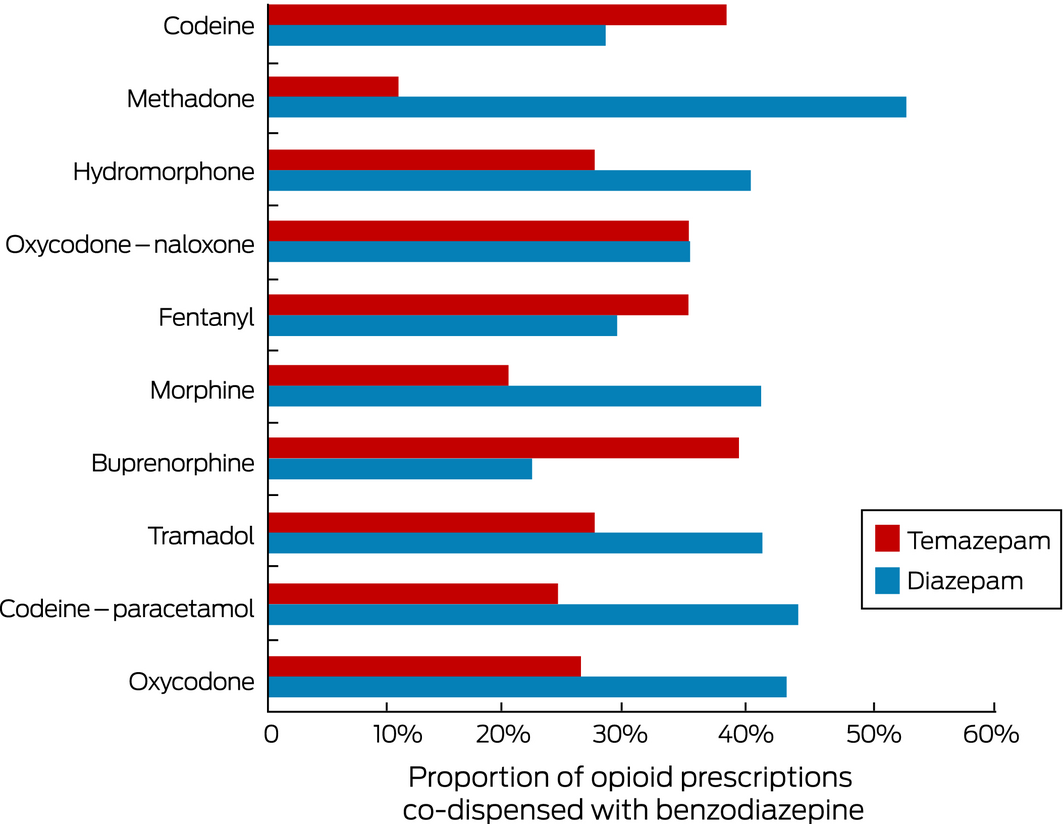
The number of people concomitantly dispensed benzodiazepines and opioids declined from 93 630 (14.8% of people dispensed opioids) in July 2012 to 91 550 (12.7%) in June 2017, a mean annual decrease of 3.4% (RR, 0.97; 95% CI, 0.96–0.97). The mean age of people dispensed both drug types in July 2017 was 59 years (standard deviation, 18 years); 14% of concomitant dispensees were aged 40 years or less, 36% 41–60 years, and 50% were over 60; 54 930 (60%) were women. The dispensing overlap was less than a week for 19 042 people (20.8%), at least 7 days but less than a month for 61 064 (66.7%), and a full month for 11 444 (12.5%). Codeine–paracetamol (26.2% of opioid medications dispensed) and oxycodone (24.9%) were the two most frequently dispensed opioid medications; diazepam (38.5%) and temazepam (33.8%) the most frequently dispensed benzodiazepines (Box 1). Concomitant dispensing of individual opioids with diazepam ranged from 22% to 53%, and with temazepam from 11% to 39% (Box 2).
The proportion of concomitant benzodiazepine dispensing to opioid users in Australia has declined since 2012 but remains substantial (12.7%), similar to the level in US studies (9.6–17%) associated with serious harms and FDA safety warnings.1,2,3 The most frequently dispensed benzodiazepine, diazepam, is long acting and has pharmacologically active metabolites, and may therefore cause excessive sedation. While 76% of opioid overdose deaths in Australia are of younger adults (25–54 years of age),5 concomitant use with benzodiazepines is frequent among older people, who are at increased risk of medication‐related adverse effects such as respiratory depression, falls, and fractures. Concerted efforts by health professionals and policy makers in Australia are clearly needed to reduce overprescribing of these medications, to identify patients at greater risk, and to implement appropriate risk mitigation strategies.
Received 21 May 2018, accepted 24 August 2018
- 1. Hwang CS KE, Kornegay CJ, Staffa JA, et al. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am J Prev Med 2016; 51: 151–160.
- 2. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 2015 49: 493–501.
- 3. Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case‐cohort study. BMJ 2015; 350: h2698.
- 4. US Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [media release]. 31 Aug 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm (viewed Sept 2018).
- 5. Roxburgh A, Hall WD, Dobbins T, et al. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend 2017; 179: 291–298.





This research was supported by a National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Post‐Marketing Surveillance of Medicines and Medical Devices grant (GNT1040938). Elizabeth Roughead is supported by an NHMRC Fellowship (GNT 1110139).
No relevant disclosures.