Abstract
Introduction: Atrial fibrillation (AF) is increasing in prevalence and is associated with significant morbidity and mortality. The optimal diagnostic and treatment strategies for AF are continually evolving and care for patients requires confidence in integrating these new developments into practice. These clinical practice guidelines will assist Australian practitioners in the diagnosis and management of adult patients with AF.
Main recommendations: These guidelines provide advice on the standardised assessment and management of patients with atrial fibrillation regarding:
- screening, prevention and diagnostic work-up;
- acute and chronic arrhythmia management with antiarrhythmic therapy and percutaneous and surgical ablative therapies;
- stroke prevention and optimal use of anticoagulants; and
- integrated multidisciplinary care.
Changes in management as a result of the guideline:
- Opportunistic screening in the clinic or community is recommended for patients over 65 years of age.
- The importance of deciding between a rate and rhythm control strategy at the time of diagnosis and periodically thereafter is highlighted. β-Blockers or non-dihydropyridine calcium channel antagonists remain the first line choice for acute and chronic rate control. Cardioversion remains first line choice for acute rhythm control when clinically indicated. Flecainide is preferable to amiodarone for acute and chronic rhythm control. Failure of rate or rhythm control should prompt consideration of percutaneous or surgical ablation.
- The sexless CHA2DS2-VA score is recommended to assess stroke risk, which standardises thresholds across men and women; anticoagulation is not recommended for a score of 0, and is recommended for a score of ≥ 2. If anticoagulation is indicated, non-vitamin K oral anticoagulants are recommended in preference to warfarin.
- An integrated care approach should be adopted, delivered by multidisciplinary teams, including patient education and the use of eHealth tools and resources where available. Regular monitoring and feedback of risk factor control, treatment adherence and persistence should occur.
Atrial fibrillation (AF) is a burdensome condition with increasing prevalence. International guidelines on the diagnosis and management of AF are available,1,2 but individual recommendations may differ, and no guidelines have previously been developed specific to the Australian population.
These guidelines have been developed by the National Heart Foundation of Australia (NHFA) and the Cardiac Society of Australia and New Zealand (CSANZ) to assist Australian clinicians in the diagnosis and management of adult patients with AF. They are informed by recent evidence interpreted by local experts to optimise application in an Australian context.
This executive summary provides important recommendations together with their strength of evidence and guidance for their implementation in clinical practice (practice points). The full clinical guidelines are available in Heart, Lung and Circulation at https://doi.org/10.1016/j.hlc.2018.06.1043.3
Method
The NHFA, in partnership with the CSANZ, appointed an expert working group comprising cardiologists (including electrophysiologists), an epidemiologist and physician, a pharmacist, nurses, a consumer, general practitioners, a neurologist, and a cardiothoracic surgeon. Three subgroups covered the topics of screening and prevention, arrhythmia management, and stroke prevention.
A reference group of representatives from key stakeholder organisations with national relevance in the provision of AF care in Australia was formed. This group provided input into the scope of the guidelines and guideline content.
A draft of the guidelines was open for a 21-day period of public consultation in April 2018 to capture stakeholder views and facilitate engagement. Appropriate governance processes were followed to ensure transparency, minimise bias, manage conflict of interest and limit other influences during guideline development.
Key evidence-based recommendations
Each recommendation is presented with a Grading of Recommendations Assessment, Development and Evaluation (GRADE) strength of recommendation and quality of evidence.4 Practice points are also provided.
Screening and prevention
-
Opportunistic point-of-care screening in the clinic or community should be conducted in people aged 65 years or more. GRADE: Strong; Evidence: Moderate.
-
Practice point: Devices that provide a medical quality electrocardiogram trace are preferred to pulse-taking or pulse-based devices for screening, because an electrocardiogram is required to confirm the diagnosis.
-
-
Pacemakers and defibrillators should be interrogated regularly for atrial high rate episodes (AHREs), and should be confirmed by atrial electrocardiogram to be AF. GRADE: Strong; Evidence: Moderate.
-
Practice point: Detection of AHREs on devices indicates a high risk of subsequent development of clinical AF.5,6 If an AHRE is detected, further assessment of stroke risk factors and surveillance for development of clinical AF should be performed.7
-
-
A transthoracic echocardiogram should be performed in all patients with newly diagnosed AF. GRADE: Strong; Evidence: Low.
-
Practice point: A transthoracic echocardiogram can identify valvular heart disease and quantify left ventricular function and atrial size. Transoesophageal echocardiography can be considered primarily where electrical or pharmacological cardioversion is indicated and the presence of intracardiac thrombus may affect timing.
-
-
Intercurrent risk factors and comorbidities, including hypertension, diabetes, heart failure, valvular heart disease and alcohol excess, should be identified and their management considered an important component of treatment in patients with AF. GRADE: Strong; Evidence: Low.
-
Practice point: The more risk factors that an individual has, the greater the likelihood that a person will develop AF and more persistent AF.8,9 With the burden of AF increasing at rates greater than those predicted by known risk factors, there has been interest in several newer risk factors,10 including obesity, sleep apnoea, physical inactivity and prehypertension.11-15 Physician-led intervention of weight and risk factor management in overweight and obese patients has been shown to lead to a marked reduction in AF burden, and to an improvement in quality of life in patients with paroxysmal AF.16
-
Arrhythmia management
-
A rhythm control or a rate control strategy should be selected, documented and communicated for all AF patients, and this strategy should be reviewed regularly. GRADE: Strong; Evidence: Low.
-
Practice point: Factors favouring rhythm over rate control include patients who are younger, more physically active and highly symptomatic; those with paroxysmal or early persistent AF; and those with left ventricular dysfunction; no severe left atrial enlargement; and those in whom adequate control of the ventricular rate is difficult to achieve. A rate control strategy may be used in preference to rhythm control in patients with minimal symptoms or in those in whom attempts at maintaining sinus rhythm are likely to be or are futile.
-
Acute rate control
-
β-Adrenoceptor antagonists or non-dihydropyridine calcium channel antagonists are recommended for acute control of the ventricular rate in haemodynamically stable patients, although caution is needed if given intravenously. GRADE: Strong; Evidence: Low.
-
Practice point: Oral administration of these agents is sufficient in many situations. A more rapid onset of action may be seen with careful administration of intravenous aliquots of metoprolol or esmolol. Intravenous verapamil must be used with extreme caution because of its strong negative inotropic effect. Digoxin may be considered in addition to the above agents, but it has a delayed onset of action and has a weak effect in terms of rate control, particularly when used as monotherapy.17 In patients with marginal haemodynamic reserve, established heart failure or other significant structural heart disease, amiodarone may be the most effective rate control option (Box 1).
-
Long term rate control
-
β-Adrenoceptor antagonists or non-dihydropyridine calcium channel antagonists should be the first line agents used for long term control of the ventricular rate.18 GRADE: Strong; Evidence: Moderate.
-
Digoxin can be useful as a second line agent or in combination with β-blockers or calcium antagonists and, if used, serum concentration should be monitored with the goal of maintaining levels < 1.2 ng/mL. Verapamil and diltiazem should not be used in the presence of left ventricular systolic dysfunction (Box 2). Amiodarone should be considered a last line option, given its toxicity profile. Membrane-active rhythm control agents (eg, flecainide or sotalol) should not be continued in patients being started on or transitioned to a long term rate control strategy.
-
Acute rhythm control
-
Electrical cardioversion should be performed urgently in haemodynamically unstable patients with AF. GRADE: Strong; Evidence: Low.
-
Electrical cardioversion can be considered — either as a first line option or when pharmacological rhythm control fails — in haemodynamically stable patients, after consideration of thromboembolic risk. GRADE: Strong; Evidence: Low.
-
Flecainide can be considered for rapid conversion to sinus rhythm, either intravenously or orally, in patients without left ventricular systolic dysfunction, moderate left ventricular hypertrophy or coronary artery disease, after consideration of thromboembolic risk. GRADE: Strong; Evidence: Moderate.
-
Practice point: There is a high spontaneous reversion rate to sinus rhythm for new onset AF within 48 hours, so a wait and watch approach with rate control may be reasonable in a mildly symptomatic patient. Flecainide or amiodarone are the recommended drugs for pharmacologic cardioversion. Flecainide results in earlier and more effective conversion to sinus rhythm when compared with amiodarone.19,20 Atrioventricular nodal blocking medication should be administered to patients before flecainide to avoid 1:1 conduction of atrial flutter. In patients with an AF duration of more than 48 hours or of unknown duration, acute rhythm control should generally not be attempted unless left atrial thrombus is excluded with transoesophageal echocardiography.
-
Long term rhythm control
-
Flecainide can be considered in the maintenance of sinus rhythm in patients without left ventricular systolic dysfunction, moderate left ventricular hypertrophy or coronary artery disease. GRADE: Strong; Evidence: High.
-
Amiodarone can be considered for maintenance of sinus rhythm as a second line agent or as a first line agent in patients with left ventricular systolic dysfunction, moderate left ventricular hypertrophy or coronary artery disease. GRADE: Strong; Evidence: High.
-
Practice point: Amiodarone has superior efficacy over other antiarrhythmic drugs or placebo in maintenance of sinus rhythm.21-25 However, amiodarone is associated with potential long term toxicities and therefore should not be a first line treatment choice (Box 3). Flecainide should be used in conjunction with an atrioventricular nodal block agent. Sotalol has modest efficacy in maintenance of sinus rhythm,21,22,26,27 and torsades de pointes occurs in about 2% of patients,28 necessitating close monitoring of the QT interval for all patients.29 β-Blockers are generally regarded as less effective than antiarrhythmic drugs in the maintenance of sinus rhythm.1,2,24
-
Percutaneous catheter atrial fibrillation ablation
-
Catheter ablation should be considered for symptomatic paroxysmal or persistent AF refractory or intolerant to at least one class I or III antiarrhythmic medication. GRADE: Strong; Evidence: High.
-
Catheter ablation can be considered for symptomatic paroxysmal or persistent AF in selected patients with heart failure with reduced ejection fraction. GRADE: Strong; Evidence: Moderate.
-
Practice point: AF ablation is an effective procedure for appropriately selected patients with symptomatic AF.30 Recent evidence demonstrates that the procedure may have a mortality benefit in patients with heart failure.31 In the discussion with the patient, it is important to emphasise that 20–30% of ablation patients will require a second procedure within the first 12 months. Major complication rates from experienced Australian institutions have been about 1%.32 In patients at increased risk of stroke, anticoagulation should be continued indefinitely, even following a successful procedure.
-
Surgical management of atrial fibrillation
-
Surgical ablation of AF to restore sinus rhythm in the context of concomitant cardiac surgery may be considered for patients with symptomatic paroxysmal, persistent or long-standing persistent AF. GRADE: Strong; Evidence: Moderate.
-
Most studies comparing coronary artery bypass grafting and concomitant surgical ablation of AF with coronary artery bypass grafting alone showed a reduction in AF recurrence, and no significant difference in morbidity or mortality.33-36
-
Stroke prevention
-
The CHA2DS2-VA score — the sexless CHA2DS2-VASc score — is recommended for predicting stroke risk in AF. GRADE: Strong; Evidence: Moderate.
-
Practice point: To avoid the cumbersome practice of selecting different CHA2DS2-VASc thresholds for males and females when recommending anticoagulation, these guidelines recommend a sexless CHA2DS2-VASc score, abbreviated as CHA2DS2-VA score (Box 4). Stroke risk factors may change over time due to ageing or development of new comorbidities. Hence, annual review of low risk patients is recommended to ensure that risk is adequately characterised to guide oral anticoagulant (OAC) therapy.
-
-
Reversible bleeding factors should be identified and corrected in AF patients for whom anticoagulation is indicated. GRADE: Strong; Evidence: Low.
-
Practice point: Patients at high risk of stroke are also at high risk of major bleeding.37 The net clinical benefit almost always favours stroke prevention over major bleeding, so bleeding risk scores should not be used to avoid anticoagulation in patients with AF. Treating reversible bleeding risk should be prioritised to minimise the bleeding rate in patients on anticoagulants (Box 5).
-
-
Oral anticoagulation therapy to prevent stroke and systemic embolism is recommended in patients with non-valvular AF whose CHA2DS2-VA score is 2 or more, unless there are contraindications to anticoagulation. GRADE: Strong; Evidence: High.
-
Oral anticoagulation therapy to prevent stroke and systemic embolism should be considered in patients with non-valvular AF whose CHA2DS2-VA score is 1. GRADE: Strong; Evidence: Moderate.
-
Oral anticoagulation therapy to prevent thromboembolism and systemic embolism is not recommended in patients with non-valvular AF whose CHA2DS2-VA score is 0. GRADE: Weak; Evidence: Moderate.
-
Practice point: Non-valvular AF refers to AF in the absence of moderate to severe mitral stenosis or mechanical heart valve. The CHA2DS2-VA score should be used to determine a threshold at which oral anticoagulation therapy is recommended (Box 6). Asymptomatic patients with AF detected on opportunistic screening have a comparable stroke risk to symptomatic patients. Patients with atrial flutter have a slightly lower stroke risk than patients with AF, but the risk still exists40 and many of these patients have episodes of AF so the same recommendations for anticoagulation apply. The stroke risk for patients with implantable devices and incidentally detected AF appears to be lower than in the general AF population, but patients with a CHA2DS2-VA score ≥ 2 should have close follow-up for development of clinical AF, with consideration of an OAC when an episode lasts for more than 24 hours.
-
-
When oral anticoagulation is initiated in a patient with non-valvular AF, a non-vitamin K OAC (NOAC; apixaban, dabigatran or rivaroxaban) is recommended in preference to warfarin. GRADE: Strong; Evidence: Moderate.
-
Antiplatelet therapy is not recommended for stroke prevention in non-valvular AF patients, regardless of stroke risk. Grade: Strong; Evidence: Moderate.
-
Practice point: Anticoagulation with warfarin reduces the risk of embolic stroke by 64% and of mortality by 26% when used in patients with non-valvular AF.41 Randomised data show that NOACs are as good as or better than warfarin in reducing stroke and systemic embolism, and that bleeding rates are less or similar to warfarin. Intracranial haemorrhage is significantly reduced with NOACs compared with warfarin. NOACs have minimal drug and food interactions, and do not need haematological monitoring, so are much easier for the patient and physician to use.42-45 International normalised ratio (INR) monitoring may be difficult in remote Australian communities, and therefore NOACs have the capacity to greatly improve anticoagulant therapy in patients with non-valvular AF. The evidence for stroke prevention with aspirin is weak, and aspirin may have bleeding rates similar to OACs.46
-
-
Point-of-care INR measurement is recommended in the primary care management of patients receiving warfarin. GRADE: Strong; Evidence: Moderate.
-
Practice point: Current point-of-care measurement of INR for warfarin therapy is most useful for patients who are generally stable and/or in acute situations where a timely result is needed to guide patient management.
-
-
Careful assessment of the bleeding and ischaemic risks (ie, stroke, new or recurrent cardiac ischaemia or infarction, and stent thrombosis) should be undertaken for patients with AF who have a long term requirement for anticoagulation for stroke prevention and require dual antiplatelet therapy after acute coronary syndrome or stent implantation (or both). GRADE: Strong; Evidence: Low.
-
Practice point: Duration of triple therapy (aspirin, P2Y12 inhibitor and OAC) should be as short as possible to minimise bleeding, while ensuring coverage of the initial period of high thrombotic risk (see Figure 8 in the full guidelines3). The risk of gastrointestinal bleeding in patients on triple therapy is likely to be reduced by concomitant administration of proton pump inhibitors.47 Where dual antiplatelet therapy is required in combination with an OAC, aspirin and clopidogrel are recommended. Where an OAC is used for AF, discontinuation of antiplatelet therapy should be considered 12 months after stent implantation, acute coronary syndrome, or both, with continuation of OAC therapy alone.
-
Integrated care
-
An integrated care approach is recommended; such an approach aims to provide patient-centred comprehensive treatment delivered by a multidisciplinary team. GRADE: Strong; Evidence: High.
-
Practice point: Integrated care focuses on three fundamental aspects: multidisciplinary teams, patient-centred care with a focus on shared decision making, and application of eHealth.48,49
-
-
All patients prescribed pharmacotherapy for the management of AF, including core rhythm control and anticoagulation therapies, should have their treatment adherence and persistence regularly monitored and supported using accessible and patient-centred strategies. GRADE: Strong; Evidence: Low.
-
Practice point: Long term persistence to OACs tends to decrease over time; about one-third to half of patients discontinue therapy within 2.5 years of initiation.50,51 Recent studies focus on improving adherence to anticoagulants via the use of electronic applications, with mixed results. Earlier studies focused on educational and behavioural interventions, but did not generate enough evidence to determine their impact.52 Regular monitoring and feedback of treatment adherence and persistence should be prioritised to optimise and standardise care and improve outcomes.
-
Box 1 – Acute rate control of atrial fibrillation with rapid ventricular response
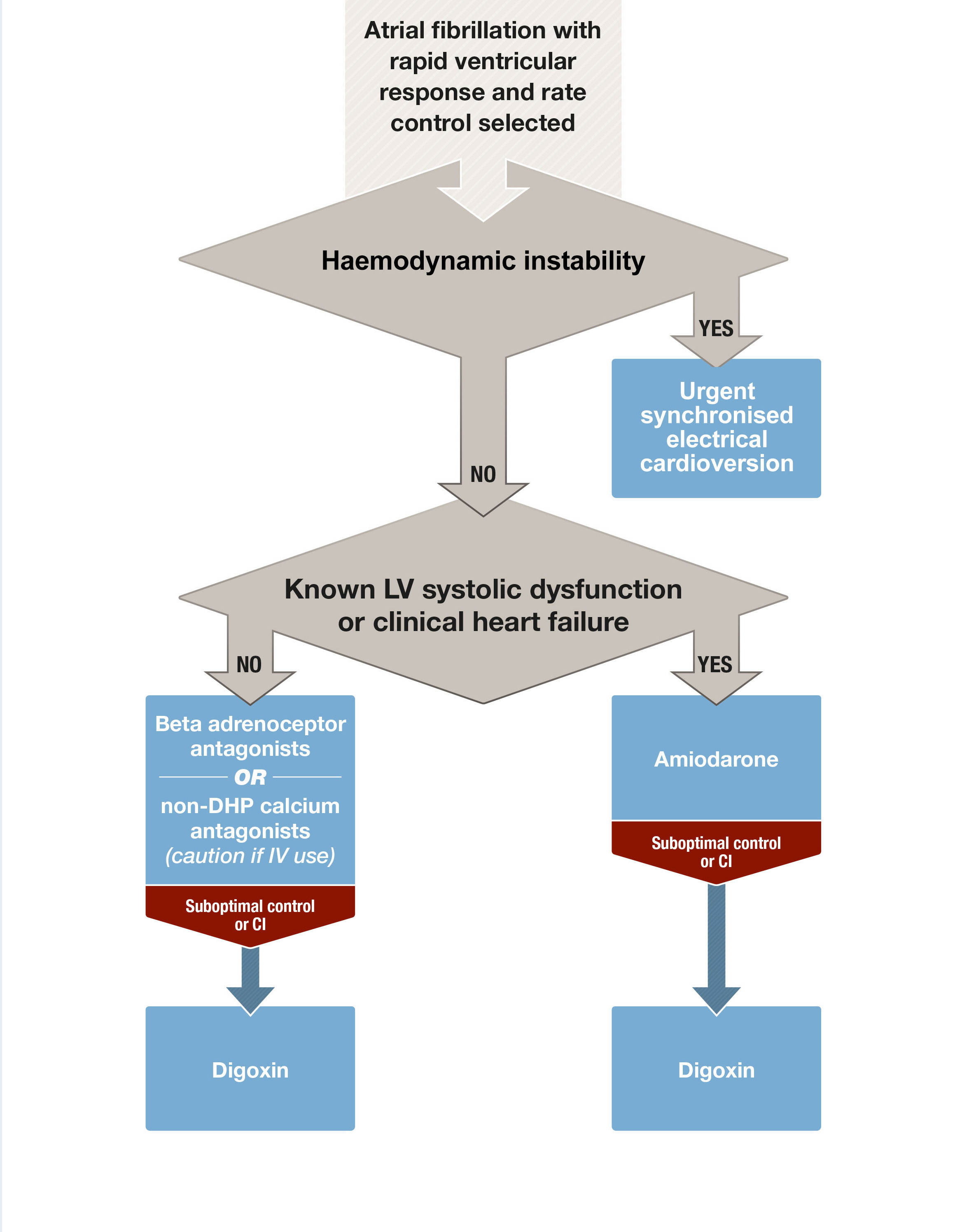
CI = contraindications; DHP = dihydropyridine; IV = intravenous; LV = left ventricular.
Box 2 – Chronic rate control of atrial fibrillation with rapid ventricular response
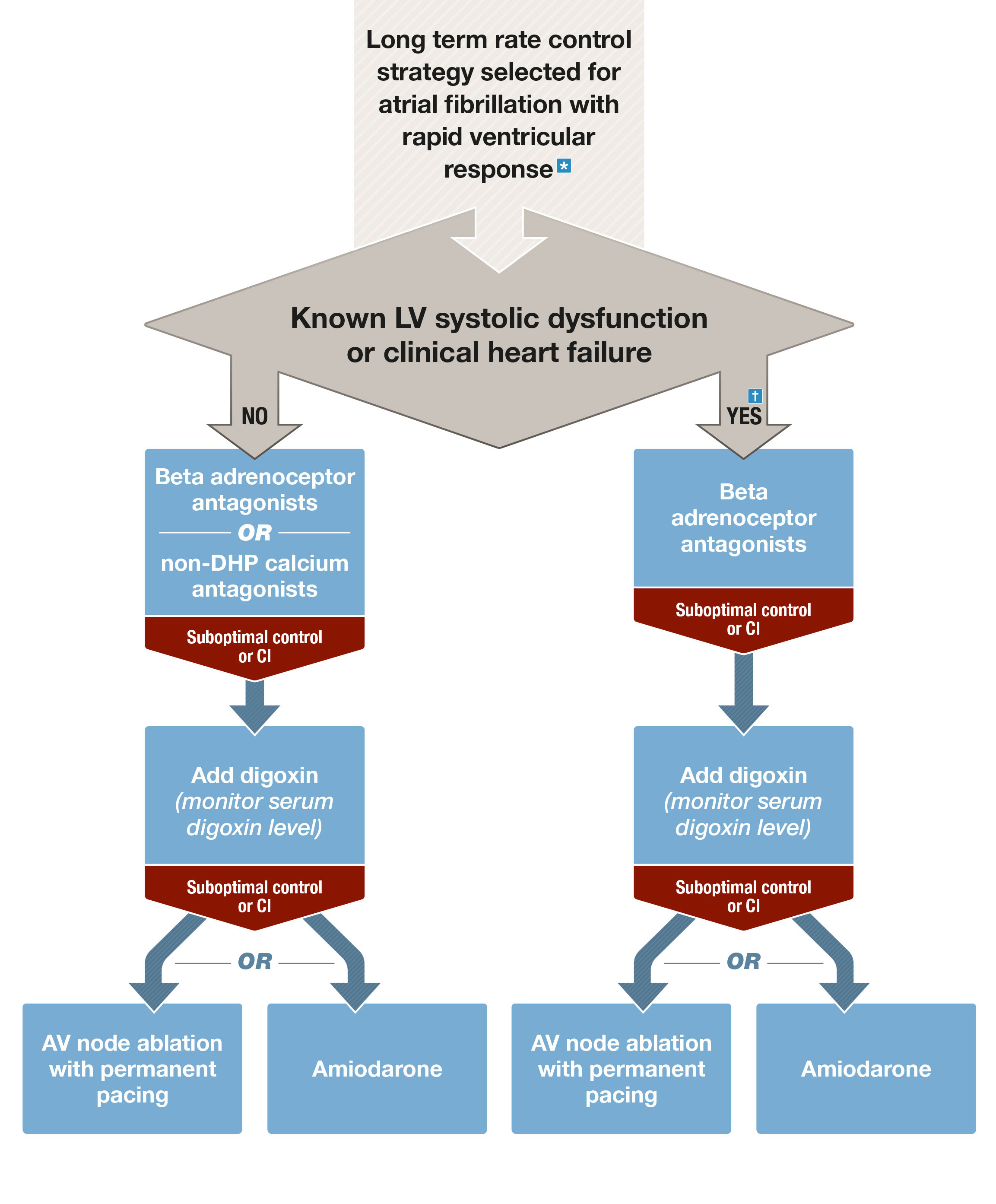
AF = atrial fibrillation; AV = atrioventricular; CI = contraindications; DHP = dihydropyridine; IV = intravenous; LV = left ventricular. * Ensure membrane-active antiarrhythmic rhythm control agents are ceased once rate control strategy adopted. † Re-evaluate the potential role of a rhythm control strategy (in particular with catheter ablation) in patients with heart failure, with the goal of reversing systolic dysfunction and improving prognosis.
Box 3 – Long term rhythm control strategies
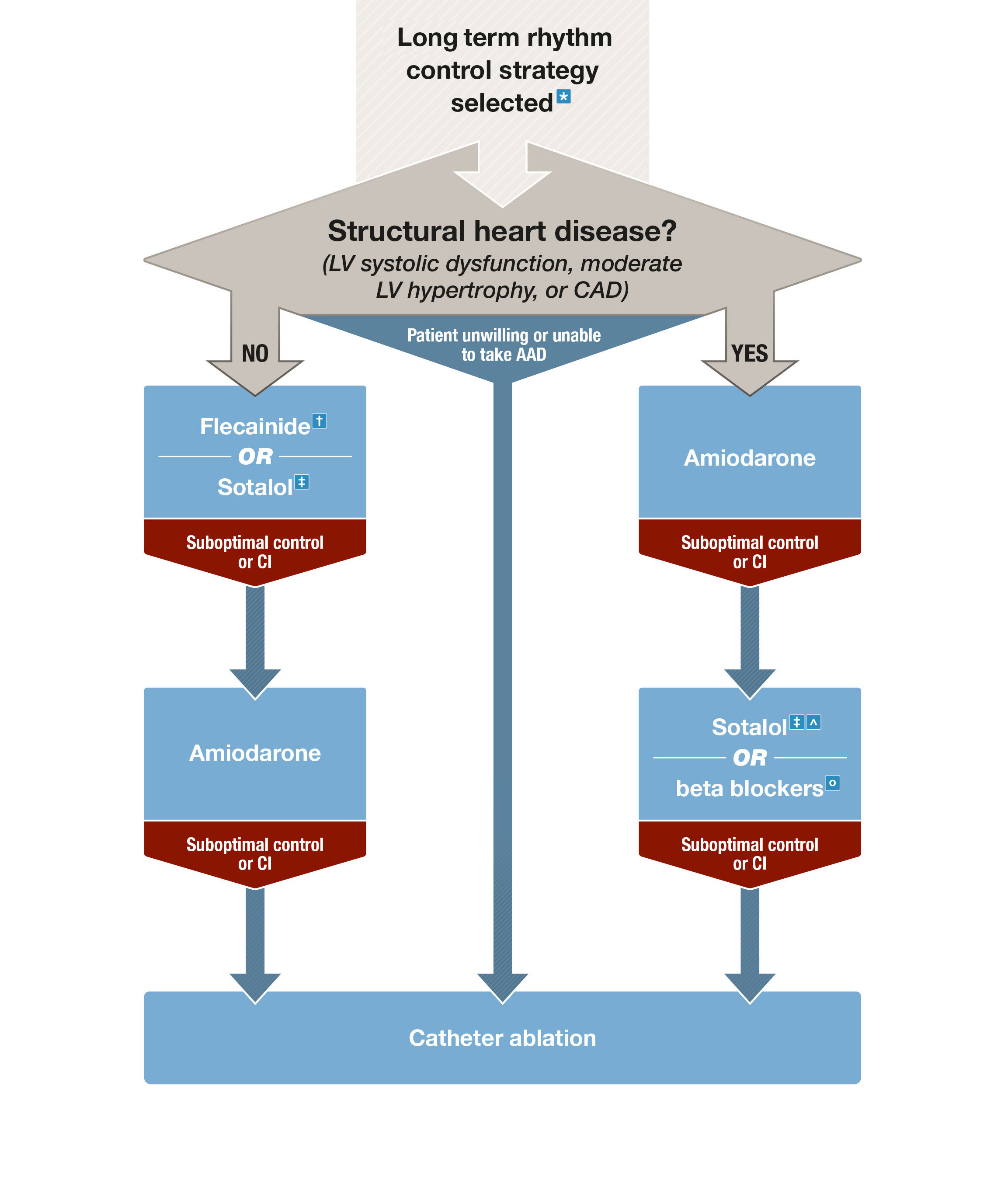
AAD = antiarrhythmic drugs; AF = atrial fibrillation; AV = atrioventricular; CAD = coronary artery disease; CI = contraindications; DHP = dihydropyridine; LV = left ventricular; LVEF = left ventricular ejection fraction. * See Table 2 in the full guidelines3 for factors favouring rhythm control strategy. † With AV nodal blocking agent. ‡ Close monitoring of QT interval recommended. ˆ May worsen heart failure, contraindicated in patients with LVEF < 40%. ○ Indicated in patients with heart failure with reduced ejection fraction, may be less effective than other AAD in maintenance of sinus rhythm.
Box 4 – Definitions and points in the CHA2DS2-VA score
|
Score |
Points |
Definition |
|||||||||||||
|
|
|||||||||||||||
|
C |
1 |
Congestive heart failure: recent signs, symptoms or admission for decompensated heart failure; this includes both HFrEF and HFpEF, or moderately to severely reduced systolic left ventricular function, whether or not there is a history of heart failure |
|||||||||||||
|
H |
1 |
History of hypertension, whether or not blood pressure is currently elevated |
|||||||||||||
|
A2 |
2 |
Age ≥ 75 years |
|||||||||||||
|
D |
1 |
Diabetes |
|||||||||||||
|
S2 |
2 |
History of prior stroke or transient ischaemic attack or systemic thromboembolism |
|||||||||||||
|
V |
1 |
Vascular disease, defined as prior myocardial infarction or peripheral arterial disease or complex aortic atheroma or plaque on imaging (if performed) |
|||||||||||||
|
A |
1 |
Age 65–74 years |
|||||||||||||
|
|
|||||||||||||||
|
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction. |
|||||||||||||||
Box 5 – Bleeding risk factors*
|
Bleeding risk factor |
Comment |
||||||||||||||
|
|
|||||||||||||||
|
Modifiable |
|
||||||||||||||
|
Hypertension (SBP > 160 mmHg) |
Blood pressure control reduces the potential risk of bleeding |
||||||||||||||
|
Labile INR (TTR < 60%) |
Consider changing to an NOAC |
||||||||||||||
|
Concomitant medications including antiplatelet agents and NSAIDs |
Minimise duration of double or triple therapy in patients with coronary disease and AF |
||||||||||||||
|
Excess alcohol (> 8 drinks/week) |
|
||||||||||||||
|
Potentially modifiable |
Correct these factors where possible |
||||||||||||||
|
Anaemia |
|
||||||||||||||
|
Impaired renal function |
Monitor, especially in situations when renal function may be affected |
||||||||||||||
|
Impaired liver function |
|
||||||||||||||
|
Frailty and falls |
Walking aids, footwear, aged care home review |
||||||||||||||
|
Non-modifiable |
|
||||||||||||||
|
Advanced age |
Stroke risk outweighs bleeding risk |
||||||||||||||
|
History of major bleeding |
|
||||||||||||||
|
Previous stroke |
Risk of recurrent stroke outweighs risk of bleeding |
||||||||||||||
|
Dialysis-dependent kidney disease |
The role of anticoagulation (warfarin only indicated) in this population is controversial |
||||||||||||||
|
Cirrhotic liver disease |
Contraindication to NOACs (patients are excluded from trials); consider advice from hepatologist |
||||||||||||||
|
Malignancy |
Individualise decisions about anticoagulation based on risk and benefit |
||||||||||||||
|
Genetic or racial variation |
Subgroup analyses from the NOAC versus warfarin RCTs suggest that, when warfarin is used, Asian patients are at higher risk of major bleeding and ICH than non-Asians; standard dose NOACs appear to be as effective in Asians as non-Asians38 |
||||||||||||||
|
|
|||||||||||||||
|
AF = atrial fibrillation; ICH = intracranial haemorrhage; INR = international normalised ratio; NOAC = non-vitamin K oral anticoagulant; NSAID = non-steroidal anti-inflammatory drug; RCT = randomised controlled trial; SBP = systolic blood pressure; TTR = time in therapeutic range. * Adapted with permission from Kirchhof et al.1 |
|||||||||||||||
Box 6 – Stroke prevention in atrial fibrillation*
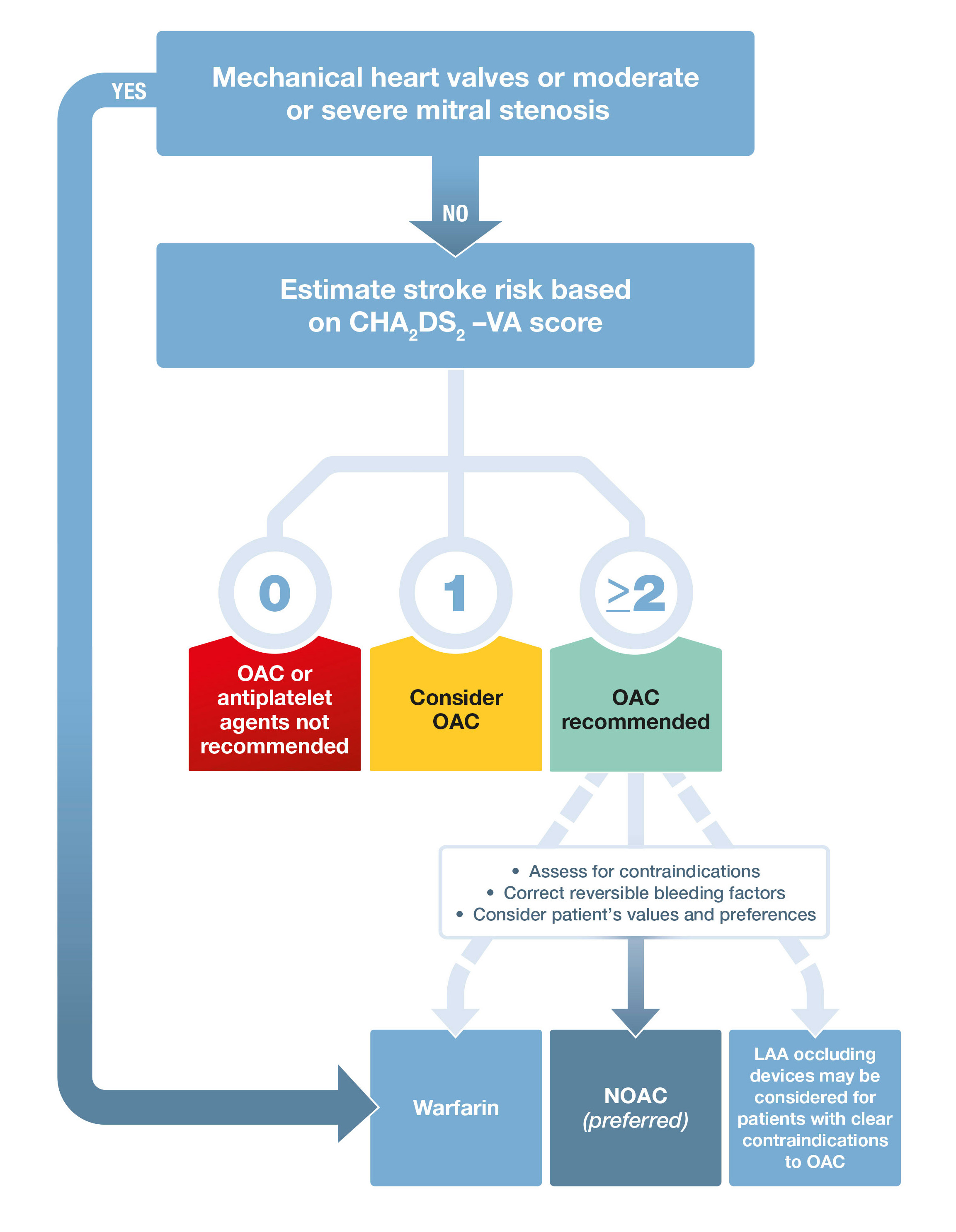
LAA = left atrial appendage; NOAC = non-vitamin K oral anticoagulant. OAC = oral anticoagulant. * Adapted with permission from Kirchhof et al.1
Provenance: Not commissioned; externally peer reviewed.
- David Brieger1
- John Amerena2
- John R Attia3,4
- Beata Bajorek5
- Kim H Chan6,7
- Cia Connell8
- Ben Freedman7
- Caleb Ferguson9,10
- Tanya Hall11
- Haris M Haqqani12
- Jeroen Hendriks13,14
- Charlotte M Hespe15
- Joseph Hung16
- Jonathan M Kalman17,18
- Prashanthan Sanders13,14
- John Worthington6
- Tristan Yan6
- Nicholas A Zwar19
- 1 Concord Repatriation General Hospital, Sydney, NSW
- 2 University Hospital Geelong, Geelong, VIC
- 3 University of Newcastle, Newcastle, NSW
- 4 John Hunter Hospital, Newcastle, NSW
- 5 UTS Sydney, Sydney, NSW
- 6 Royal Prince Alfred Hospital, Sydney, NSW
- 7 University of Sydney, Sydney, NSW
- 8 National Heart Foundation of Australia, Melbourne, VIC
- 9 Western Sydney University, Sydney, NSW
- 10 Blacktown and Mount Druitt Hospital, Sydney, NSW
- 11 Hearts4heart, Melbourne, VIC
- 12 Prince Charles Hospital, Brisbane, QLD
- 13 Royal Adelaide Hospital, Adelaide, SA
- 14 University of Adelaide, Adelaide
- 15 University of Notre Dame Australia, Sydney, NSW
- 16 Sir Charles Gairdner Hospital, Perth, WA
- 17 University of Melbourne, Melbourne, VIC
- 18 Royal Melbourne Hospital, Melbourne, VIC
- 19 University of Wollongong, Wollongong, NSW
A full conflict of interest register is available at:
https://www.heartfoundation.org.au/for-professionals/clinical-information/atrial-fibrillation
- 1. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893-2962.
- 2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23): 2071-2104.
- 3. Brieger D, Amarena J, Attia J, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018; https://doi.org/10.1016/j.hlc.2018.06.1043.
- 4. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (viewed Feb 2018).
- 5. Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018; 39: 1407-1415.
- 6. Freedman B, Boriani G, Glotzer TV, et al. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017; 14: 701-714.
- 7. Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options – a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012; 14: 8-27.
- 8. Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011; 91: 265-325.
- 9. Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011; 107: 85-91.
- 10. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114: 119-125.
- 11. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49: 565-571.
- 12. Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol 2010; 55: 2319-2327.
- 13. Lau DH, Middeldorp ME, Brooks AG, et al. Aortic stiffness in lone atrial fibrillation: a novel risk factor for arrhythmia recurrence. PLoS One 2013; 8: e76776.
- 14. Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008; 118: 800-807.
- 15. Huxley RR, Misialek JR, Agarwal SK, et al. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol 2014; 7: 620-625.
- 16. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013; 310: 2050-2060.
- 17. Schreck, DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med 1997; 29: 135-140.
- 18. Segal JB, McNamara RL, Miller MR, et al. The evidence regarding the drugs used for ventricular rate control. J Fam Pract 2000; 49: 47-59.
- 19. Capucci A, Lenzi T, Boriani G, et al. Effectiveness of loading oral flecainide for converting recent-onset atrial fibrillation to sinus rhythm in patients without organic heart disease or with only systemic hypertension. Am J Cardiol 1992; 70: 69-72.
- 20. Chevalier P, Durand-Dubief A, Burri H, et al. Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol 2003; 41: 255-262.
- 21. Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352: 1861-1872.
- 22. Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000; 342: 913-920.
- 23. The Atrial Fibrillation Follow-up Investigation of Rhythm Management Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347: 1825-1833.
- 24. Lafuente-Lafuente C, Valembois L, Bergmann JF, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2015; (3): CD005049.
- 25. McNamara RL, Tamariz LJ, Segal JB, et al. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med 2003; 139: 1018-1033.
- 26. Benditt DG, Williams JH, Jin J, et al. Maintenance of sinus rhythm with oral d,l-sotalol therapy in patients with symptomatic atrial fibrillation and/or atrial flutter. d,l-Sotalol Atrial Fibrillation/Flutter Study Group. Am J Cardiol 1999; 84: 270-277.
- 27. Fetsch T, Bauer P, Engberding R, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J 2004; 25: 1385-1394.
- 28. MacNeil DJ, Davies RO, Deitchman D. Clinical safety profile of sotalol in the treatment of arrhythmias. Am J Cardiol 1993; 72(4): 44a-50a.
- 29. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6: 479-487.
- 30. Kalla M, Sanders P, Kalman JM, et al. Radiofrequency catheter ablation for atrial fibrillation: approaches and outcomes. Heart Lung Circ 2017; 26: 941-949.
- 31. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417-427.
- 32. Voskoboinik A, Sparks PB, Morton JB, et al. Low rates of major complications for radiofrequency ablation of atrial fibrillation maintained over 14 years: a single centre experience of 2750 consecutive cases. Heart Lung Circ 2018; 27: 976-983.
- 33. Cherniavsky A, Kareva Y, Pak I, et al. Assessment of results of surgical treatment for persistent atrial fibrillation during coronary artery bypass grafting using implantable loop recorders. Interact Cardiovasc Thorac Surg 2014; 18: 727-731.
- 34. Ad N, Henry L, Hunt S, et al. Do we increase the operative risk by adding the Cox Maze III procedure to aortic valve replacement and coronary artery bypass surgery? J Thorac Cardiovasc Surg 2012; 143: 936-944.
- 35. Damiano RJ Jr, Gaynor SL, Bailey M, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg 2003; 126: 2016-2021.
- 36. Geidel S, Lass M, Krause K, et al. Persistent atrial fibrillation ablation concomitant to coronary surgery. Thorac Cardiovasc Surg 2011; 59: 207-212.
- 37. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol 2015; 38: 555-561.
- 38. Chiang C-E, Wu T-J, Ueng K-C, et al. 2016 Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formosan Med Assoc 2016; 115: 893-952.
- 39. Goldsmith K, Balabanski A, Giarola B, et al. RACP trainee awards for excellence in the field of adult medicine. Intern Med J 2017; 47: 7.
- 40. Al-Kawaz M, Omran SS, Parikh NS, et al. Comparative risks of ischemic stroke in atrial flutter versus atrial fibrillation. J Stroke Cerebrovasc Dis 2018; 27: 839-844.
- 41. Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med 2007; 147: 590-592.
- 42. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139-1151.
- 43. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981-992.
- 44. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883-891.
- 45. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955-962.
- 46. Stroke Prevention in Atrial Fibrillation Investigators. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994; 343: 687-691.
- 47. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363: 1909-1917.
- 48. Guo Y, Chen Y, Lane D, et al. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF app trial. Am J Med 2017; 130: 1388-1396.e6.
- 49. Pandya E, Bajorek BV. Assessment of Web-based education resources informing patients about stroke prevention in atrial fibrillation. J Clin Pharm Therapeut 2016; 41: 667-676.
- 50. Simons LA, Ortiz M, Freedman B, et al. Medium- to long-term persistence with non-vitamin-K oral anticoagulants in patients with atrial fibrillation: Australian experience. Curr Med Res Opin 2017; 33: 1337-1341.
- 51. Abdou JK, Auyeung V, Patel JP, et al. Adherence to long-term anticoagulation treatment, what is known and what the future might hold. Br J Haematol 2016; 174: 30-42.
- 52. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev 2013; (6): CD008600.





Oliver Frank
People in this age range visit their GPs 11.8 times each year (1). At how many of these nearly monthly consultations do the authors recommend that GPs perform an electrocardiogram?
1. Australian Institute of Health and Welfare. My Healthy Communities: GP attendances, age-standardised 2016–17. Available from: https://www.myhealthycommunities.gov.au/national/mbs0002
Competing Interests: No relevant disclosures.
Dr Oliver Frank
Oakden Medical Centre