The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have developed new guidelines to assist Australian clinicians in the care of adult patients with heart failure (HF). The guidelines are based on current evidence, and replace the 2011 guidelines for the prevention, detection and management of chronic HF in Australia.1
This executive summary provides important recommendations together with their strength of evidence and guidance for their implementation in clinical practice (practice points). The full clinical guidelines are available in Heart, Lung and Circulation at https://doi.org/10.1016/j.hlc.2018.06.1042.2
Definition of heart failure
HF is a complex clinical syndrome with typical symptoms and signs that generally occur on exertion but may also occur at rest (particularly when recumbent). HF is secondary to an abnormality of cardiac structure or function that impairs the ability of the heart to fill with blood at normal pressure or eject blood sufficient to fulfil the needs of the metabolising organs. Following clinical diagnosis, HF is generally categorised according to whether it is associated with a reduced left ventricular ejection fraction (LVEF) below 50% (heart failure with reduced ejection fraction [HFrEF]) or preserved LVEF of 50% or more (heart failure with preserved ejection fraction [HFpEF]) (Box 1).
Method
The National Heart Foundation of Australia, in partnership with the Cardiac Society of Australia and New Zealand, appointed an expert writing group. The HF guideline development working group comprised an executive and four writing groups covering the topics of diagnosis; pharmacological management; devices and surgery; and non-pharmacological management. The working group comprised a broad mix of health professionals, including cardiologists (including an electrophysiologist), nurses, general practitioners, a clinical pharmacologist and general physician, an exercise health and professional epidemiologist, and a consumer representative.
In addition, a reference group including representatives from stakeholder groups, potential endorsing organisations and regional experts provided input into the scope and content of the guideline.
A draft of the guideline was open for a 21-day period of public consultation in April 2018 to capture stakeholder views and facilitate engagement. Appropriate governance processes were followed to ensure transparency, minimise bias, manage conflict of interest and limit other influences during guideline development.
Key evidence-based recommendations
Each recommendation is presented with a Grading of Recommendations Assessment, Development and Evaluation (GRADE) strength of recommendation and quality of evidence.3 Practice points are also provided.
Prevention of heart failure
-
Blood pressure4 and lipid5 lowering according to published guidelines is recommended, to decrease the risk of cardiovascular events and the risk of developing HF. GRADE: Strong; Evidence: High.
-
Sodium–glucose cotransporter 2 inhibitors are recommended in patients with type 2 diabetes mellitus associated with cardiovascular disease and insufficient glycaemic control despite metformin, to decrease the risk of cardiovascular events and decrease the risk of HF hospitalisation.6 GRADE: Strong; Evidence: High.
-
Angiotensin-converting enzyme (ACE) inhibitors are recommended in patients with left ventricular systolic dysfunction, to decrease the risk of developing HF.7 GRADE: Strong; Evidence: High.
Diagnosis of heart failure
-
Plasma B-type natriuretic peptide (BNP) or N-terminal proBNP levels are recommended for diagnosis in patients with suspected HF when the diagnosis is uncertain.8 GRADE: Strong; Evidence: High.
-
A transthoracic echocardiogram is recommended in patients with suspected HF, to improve diagnostic accuracy, and in patients with a new diagnosis of HF, to assess cardiac structure and function (including the measurement of LVEF), assist in classification and therefore guide management.9 GRADE: Strong; Evidence: Low.
-
Practice point: The diagnostic work-up of a patient with suspected HF is summarised in Box 2. The single most useful investigation is the echocardiogram. However, if the diagnosis is unclear and an echocardiogram cannot be arranged in a timely fashion, measurement of either plasma BNP or N-terminal proBNP has been shown to improve diagnostic accuracy.2
-
Practice point: Evaluation of coronary arteries should be guided by the presence or absence of symptoms of coronary artery disease and the pre-test probability of coronary artery disease.
-
Practice point: Box 3 lists red flags where early specialist referral may be considered.
-
Management of heart failure
The management of acute HF should be guided by the patient’s vital signs, oxygen saturation, and the presence or absence of congestion and hypoperfusion. Management includes intravenous diuretics in most patients accompanied by the selected use of oxygen therapy (if hypoxaemic), positive pressure ventilation, vasodilators and inotropes.2 Effective long term management of HF is key to decreasing hospitalisation and improving survival. Although a number of evidence-based interventions exist for HFrEF (Box 4 and Box 5), none have been shown to reduce mortality in HFpEF.
Pharmacological management of chronic heart failure
-
An ACE inhibitor is recommended in all patients with HFrEF associated with an LVEF ≤ 40%, unless contraindicated or not tolerated, to decrease mortality and decrease hospitalisation.10 GRADE: Strong; Evidence: High.
-
A β-blocker (specifically bisoprolol, carvedilol, controlled or extended release metoprolol or nebivolol) is recommended in all patients with HFrEF associated with an LVEF ≤ 40% unless contraindicated or not tolerated, and once stabilised with no or minimal clinical congestion on physical examination, to decrease mortality and decrease hospitalisation.11-14 GRADE: Strong; Evidence: High.
-
A mineralocorticoid receptor antagonist (MRA) is recommended in all patients with HFrEF associated with an LVEF ≤ 40% unless contraindicated or not tolerated, to decrease mortality and decrease hospitalisation for HF.15,16 GRADE: Strong; Evidence: High.
-
An angiotensin receptor blocker (ARB) is recommended in patients with HFrEF associated with an LVEF ≤ 40% if an ACE inhibitor is contraindicated or not tolerated, to decrease the combined endpoint of cardiovascular mortality and HF hospitalisation.17 GRADE: Strong; Evidence: Moderate.
-
An angiotensin receptor neprilysin inhibitor (ARNI) is recommended as a replacement for an ACE inhibitor (with at least a 36-hour washout window) or an ARB in patients with HFrEF associated with an LVEF ≤ 40% despite receiving maximally tolerated or target doses of an ACE inhibitor (or ARB) and a β-blocker (unless contraindicated), with or without an MRA, to decrease mortality and decrease hospitalisation.18 GRADE: Strong; Evidence: High.
-
Ivabradine should be considered in patients with HFrEF associated with an LVEF ≤ 35% and with a sinus rate ≥ 70 bpm, despite receiving maximally tolerated or target doses of an ACE inhibitor (or ARB) and a β-blocker (unless contraindicated), with or without an MRA, to decrease the combined endpoint of cardiovascular mortality and HF hospitalisation.19 GRADE: Strong; Evidence: High.
-
A diuretic should be considered in patients with HF and clinical symptoms, or signs of congestion, to improve symptoms and manage congestion.20 GRADE: Strong; Evidence: Very low.
-
Unless a reversible cause has been corrected, neurohormonal antagonists (ACE inhibitors or ARBs or ARNIs, β-blockers and MRAs) should be continued at target doses in patients with HF associated with a recovered or restored ejection fraction, to decrease the risk of recurrence.21 GRADE: Strong; Evidence: Low.
-
Practice point: Aim for the target doses used in the randomised controlled trials (RCTs) that showed the benefits of these drugs. Most of the RCTs were conducted in patients with HF associated with an LVEF < 35–40%; however, post hoc analyses of patients with HF associated with a mild reduction in LVEF (LVEF, 41–49%) enrolled in RCTs have reported similar benefits with β-blockers and drugs that antagonise the renin–angiotensin–aldosterone system.22-24
-
Practice point: Patients with HFpEF are generally older with multiple comorbidities. The main aims of treatment are to improve symptoms and quality of life and decrease hospitalisation. Although the evidence for neurohormonal antagonists is less robust, these agents are often used to manage comorbidities. Low dose spironolactone may be considered to decrease HF hospitalisation.25
-
Non-pharmacological management
-
Referral to a multidisciplinary HF disease management program is recommended in patients with HF associated with high risk features, to decrease mortality and rehospitalisation.26 GRADE: Strong; Evidence: High.
-
In areas where access to a face-to-face multidisciplinary HF disease management program after discharge is limited, patients should be followed up with a multidisciplinary telemonitoring or telephone support program.27 GRADE: Strong; Evidence: Moderate.
-
Practice point: These programs should focus on high risk patients, especially those recently discharged after hospitalisation for HF. Patient and carer education, including self-management, should commence soon after diagnosis, be patient-centred appropriate to their level of health literacy, and be revised continually for life.
-
-
Nurse-led medication titration is recommended in patients diagnosed with HFrEF who have not achieved maximum tolerated doses of ACE inhibitors, ARBs, ARNIs, β-blockers or MRAs, to decrease hospitalisation.28 GRADE: Strong; Evidence: High.
-
Regular performance of up to moderate intensity (ie, breathe faster but hold conversation) continuous exercise is recommended in patients with stable chronic HF, particularly those with reduced LVEF, to improve physical functioning and quality of life and to decrease hospitalisation.29 GRADE: Strong; Evidence: High.
-
Practice point: Exercise can be considered as soon as practical in clinically stable patients. An initial period of supervision may be warranted to verify individual responses and tolerability.
-
Devices, surgery and percutaneous procedures
-
Cardiac resynchronisation therapy (CRT) is recommended in patients with HFrEF associated with sinus rhythm, an LVEF ≤ 35% and a QRS duration ≥ 150 ms despite optimal medical therapy, to decrease mortality, decrease hospitalisation for HF, and improve symptoms.30,31 GRADE: Strong; Evidence: High.
-
CRT should be considered in patients with HFrEF associated with sinus rhythm, an LVEF ≤ 35% and a QRS duration of 130–149 ms despite optimal medical therapy, to decrease mortality, decrease hospitalisation for HF, and improve symptoms.30 GRADE: Strong; Evidence: Moderate.
-
CRT should be considered in patients with HFrEF associated with an LVEF of ≤ 50% accompanied by high grade atrioventricular block requiring pacing, to decrease hospitalisation for HF.32 GRADE: Weak; Evidence: Moderate.
-
CRT is contraindicated in patients with a QRS duration < 130 ms, because of lack of efficacy and possible harm.33 GRADE: Strong Against; Evidence: Moderate.
-
Practice point: Resynchronisation of ventricular contraction is achieved by pacing both the left and the right ventricles simultaneously. The benefit is greater in patients with a broader QRS duration,30,31 and in some studies for left bundle branch block morphology and prolonged PR interval.31,34 If CRT is performed in patients in atrial fibrillation (AF), measures are required to ensure at least 92% biventricular capture.35
-
-
An implantable cardioverter defibrillator (ICD) should be considered as a primary prevention indication in patients with HFrEF associated with ischaemic heart disease and an LVEF ≤ 35%, to decrease mortality.36,37 GRADE: Strong; Evidence: Moderate.
-
An ICD may be considered as a primary prevention indication in patients with HFrEF associated with dilated cardiomyopathy and an LVEF ≤ 35%, to decrease mortality.37-39 GRADE: Weak; Evidence: Low.
-
Coronary artery bypass graft surgery should be considered in patients with HFrEF associated with ischaemic heart disease and an LVEF ≤ 35% if they have surgically correctable coronary artery disease, to improve symptoms (eg, relief of angina and HF symptoms) and decrease morbidity and long term mortality.40 GRADE: Strong; Evidence: Moderate.
-
Practice point: The benefits must be balanced against the short term morbidity and mortality risk related to coronary artery bypass graft surgery. Factors unrelated to the severity of HF — including age, frailty and comorbidities — are important contributors to surgical risk.
-
-
Surgical aortic valve replacement is recommended in patients with severe aortic stenosis or severe aortic regurgitation and HF in the absence of major comorbidities or frailty, to improve symptoms and decrease mortality.41 GRADE: Strong; Evidence: Low.
-
Transcatheter aortic valve implantation should be considered in patients with severe aortic stenosis and HF at intermediate to high operative mortality risk, or considered inoperable for surgical aortic valve replacement, and who are deemed suitable for transcatheter aortic valve implantation following assessment by a heart team, to improve symptoms and decrease mortality.42-45 GRADE: Strong; Evidence: Moderate.
-
Referral to a specialist centre for consideration of ventricular assist device implantation should be considered in patients with intractable, severe HF despite guideline-directed medical and pacemaker therapy, and who do not suffer from major comorbidities, to decrease mortality.46 GRADE: Strong; Evidence: Moderate.
-
Practice point: Timing of implantation of ventricular assist devices and patient selection are critical to achieving a successful outcome. Longer term harms including disabling stroke, bleeding and infection remain major limitations.
-
-
Referral for heart transplant assessment should be considered in patients with HF associated with intractable New York Heart Association class III–IV symptoms who have exhausted all alternative therapies and who do not have overt contraindications, to decrease mortality.47 GRADE: Strong; Evidence: Low.
Comorbidities in heart failure
-
Pharmacological therapy aiming for a resting ventricular rate of 60–100 bpm should be considered in patients with HF associated with AF and a rapid ventricular response.48,49 GRADE Strong; Evidence: Low.
-
Practice point: β-Blockers and/or digoxin are generally favoured for ventricular rate control. Consider non-dihydropyridine calcium entry blockers in patients with HFpEF to control the ventricular rate of AF; however, these drugs should be avoided in patients with HFrEF.
-
-
Catheter ablation for AF (either paroxysmal or persistent) should be considered in patients with HFrEF associated with an LVEF ≤ 35%, who present with recurrent symptomatic AF, to decrease mortality and hospitalisation for HF.49,50 GRADE: Strong; Evidence: Moderate.
-
Practice point: Consider oral amiodarone in patients with HF associated with AF, to facilitate attainment and maintenance of sinus rhythm (with or without electrical cardioversion), improve symptoms, or guide decisions regarding the need for more invasive approaches (eg, AF catheter ablation or atrioventricular node ablation).
-
-
Adaptive servoventilation is not recommended in patients with HFrEF and predominant central sleep apnoea because of increased all-cause and cardiovascular mortality.51 GRADE: Strong Against; Evidence: Moderate.
-
Practice point: Although clinicians may consider positive pressure ventilation to improve quality of life and decrease sleepiness in patients with predominant obstructive sleep apnoea, the primary aim in patients with predominant central sleep apnoea should be to treat the HF.
-
-
Erythropoietin should not be used routinely for the treatment of anaemia in patients with HF, because of an increased risk of thromboembolic adverse events.52 GRADE: Strong Against; Evidence: Moderate.
-
In patients with HFrEF associated with persistent symptoms despite optimised therapy, iron studies should be performed and, if the patient is iron deficient (ie, ferritin < 100 μg/L, or ferritin 100–300 μg/L with transferrin saturation < 20%), intravenous iron should be considered, to improve symptoms and quality of life.53 GRADE: Strong; Evidence: Moderate.
-
Practice point: If iron deficiency is diagnosed, one should consider investigation for gastrointestinal pathology, including peptic ulcer and malignancy (especially if also anaemic). Intravenous ferric carboxymaltose was evaluated in most of the RCTs, usually involving one to two doses between 500 mg and 1000 mg. Re-check iron studies after 4 months.
-
Palliative care in heart failure
-
Referral to palliative care should be considered in patients with advanced HF to alleviate end-stage symptoms, improve quality of life and decrease rehospitalisation. Involvement of palliative care should be considered early in the trajectory towards end-stage HF.54 GRADE: Strong; Evidence: High.
-
Practice point: In patients with an ICD, discussions concerning deactivation should occur between the patient, family and cardiologist. Patients should be encouraged to have an advanced care plan, regardless of clinical status and soon after diagnosis.
-
Box 1 – Heart failure diagnostic criteria
|
|
|||||||||||||||
|
Heart failure with reduced ejection fraction
|
|||||||||||||||
|
|
|||||||||||||||
|
*If LVEF mildly reduced (LVEF, 41–49%), additional criteria required (eg, signs of heart failure; diastolic dysfunction with high filling pressure demonstrated by invasive means or echocardiography or biomarker testing). BNP = B-type natriuretic peptide; LV = left ventricular; LVEF = left ventricular ejection fraction; NT = N-terminal. |
|||||||||||||||
Box 2 – Diagnostic work-up of a patient with suspected heart failure
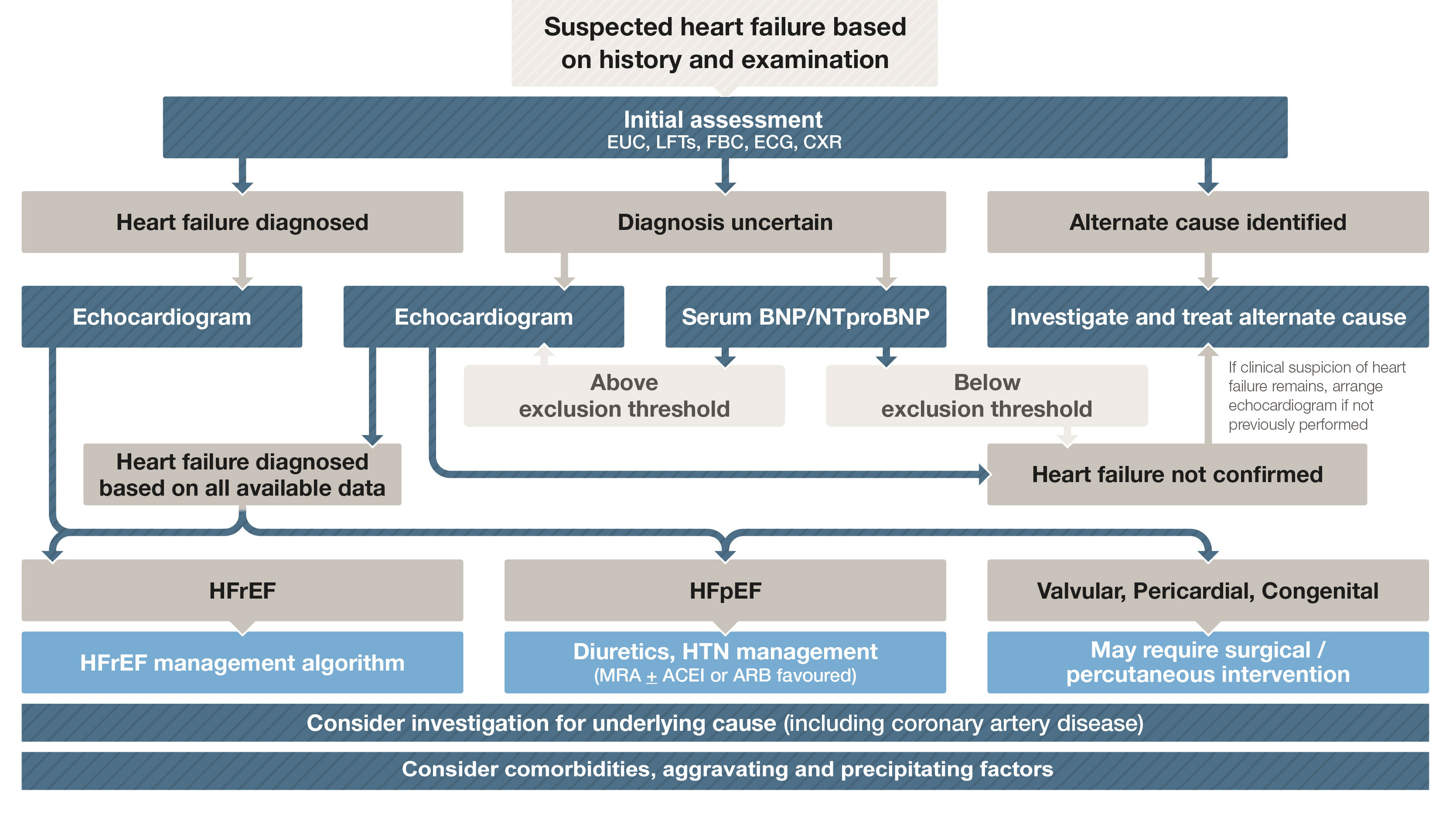
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; CXR = chest x-ray; ECG = electrocardiogram; EUC = electrolytes/urea/creatinine; FBC = full blood count; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HTN = hypertension; LFTs = liver function tests; MRA = mineralocorticoid receptor antagonist; NTproBNP = N-terminal pro B-type natriuretic peptide. Adapted with permission from Tomlinson S, Atherton JJ. Heart failure – the crucial role of the GP. Medicine Today 2018; 19: 19-27.
Box 3 – When to consider early referral in the community setting (red flags)
Symptoms
- Orthopnoea
- Paroxysmal nocturnal dyspnoea
- Syncope
- Ischaemic chest pain
Signs
- Tachycardia (heart rate > 100 bpm)
- Bradycardia (heart rate < 40 bpm)
- Hypotension (systolic blood pressure < 90 mmHg)
- Hypoxaemia
- Gallop rhythm
- Significant heart murmur
Investigations
- Evidence of ischaemia or infarction on 12-lead electrocardiogram
- Pulmonary oedema on chest x-ray
- Raised cardiac troponin level
- Moderate or severe valvular heart disease on echocardiography
- Left ventricular ejection fraction ≤ 40%
- Ischaemia on stress testing
Box 4 – Evidence summary for management of heart failure with reduced ejection fraction2
|
Treatment effect |
All patients |
Selected patients |
|||||||||||||
|
Strong recommendation |
Weak recommendation |
||||||||||||||
|
|
|||||||||||||||
|
Decrease morbidity/mortality |
ACEI (or ARB*) |
Switch ACEI or ARB to ARNI (LVEF ≤ 40%) |
ICD (DCM, LVEF ≤ 35%) |
||||||||||||
|
Improve symptoms |
|
Diuretics (congested) |
Digoxin (refractory symptoms) |
||||||||||||
|
|
|||||||||||||||
|
ACEI = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; CABG = coronary artery bypass graft surgery; CRT = cardiac resynchronisation therapy; DCM = dilated cardiomyopathy; ICD = implantable cardioverter defibrillator; IHD = ischaemic heart disease; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; SR = sinus rhythm; VAD = ventricular assist device. *ARB should only be used if ACEI is contraindicated or not tolerated. † Carvedilol, bisoprolol, metoprolol succinate, nebivolol. |
|||||||||||||||
Box 5 – Management of patients with heart failure with reduced ejection fraction (HFrEF)*
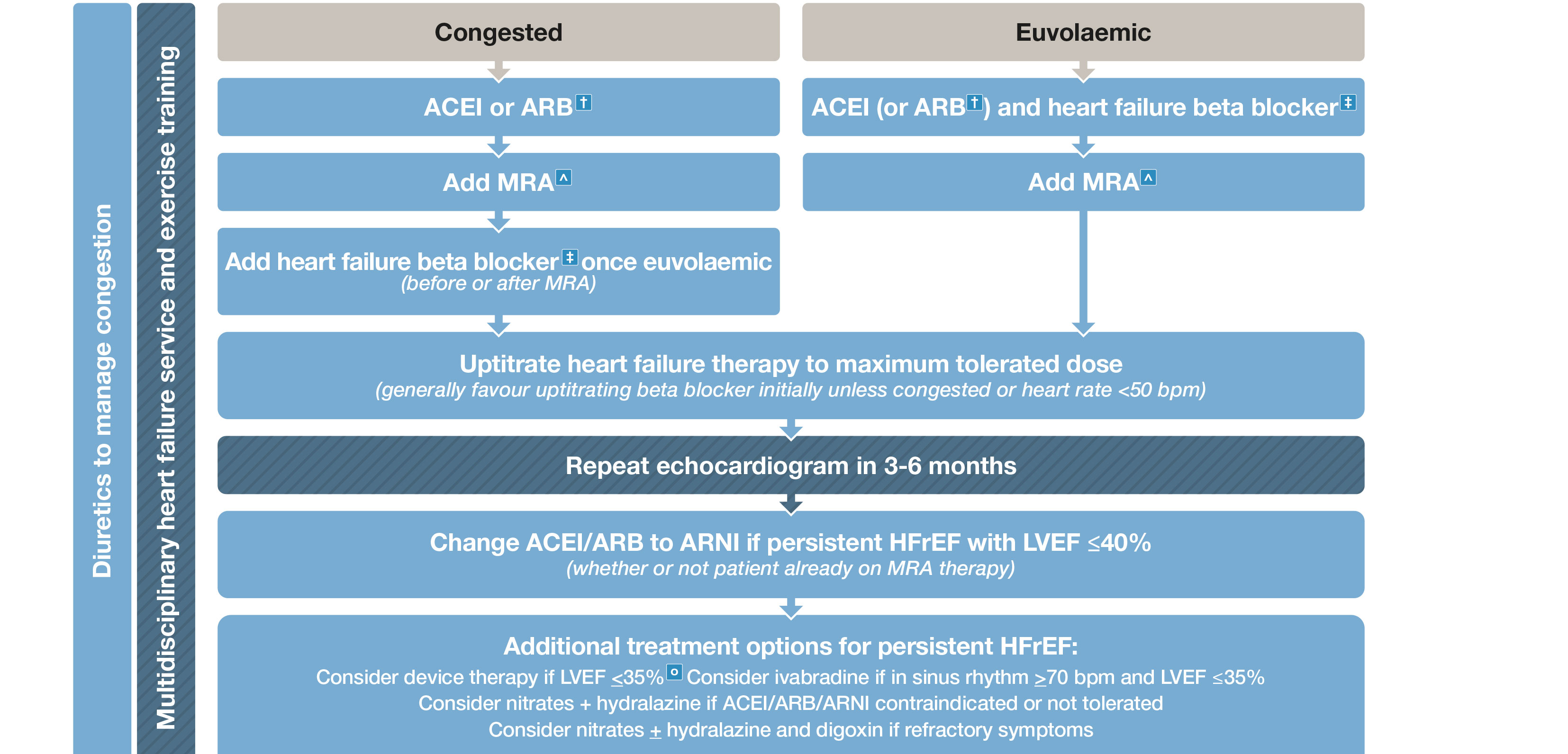
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; CRT = cardiac resynchronisation therapy; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist. * HFrEF refers to patients with symptoms ± signs of heart failure associated with and LVEF < 50% (unless otherwise specified). † ARB should only be used if ACEI is contraindicated or not tolerated. ‡ Carvedilol, bisoprolol, metoprolol succinate, nebivolol. ˆ Commencing MRA usually avoided if serum K > 5 mmol/L or CrCl < 30 mL/m. ○ ICD and/or CRT. Adapted with permission from Tomlinson S, Atherton JJ. Heart failure – the crucial role of the GP. Medicine Today 2018; 19: 19-27.
Provenance: Not commissioned; externally peer reviewed.
- John J Atherton1
- Andrew Sindone2
- Carmine G De Pasquale3
- Andrea Driscoll4,5
- Peter S MacDonald6
- Ingrid Hopper7
- Peter Kistler8
- Tom G Briffa9
- James Wong10
- Walter P Abhayaratna11
- Liza Thomas12
- Ralph Audehm13
- Phillip J Newton14
- Joan O'Loughlin15
- Cia Connell16
- Maree Branagan16
- 1 Royal Brisbane and Women's Hospital and University of Queensland, Brisbane, QLD
- 2 Concord Repatriation General Hospital, Sydney, NSW
- 3 Flinders Medical Centre, Flinders University, Adelaide, SA
- 4 Deakin University, Melbourne, VIC
- 5 Austin Health, Melbourne, VIC
- 6 St Vincent's Hospital, Sydney, NSW
- 7 Monash University, Melbourne, VIC
- 8 The Alfred Hospital, Melbourne
- 9 University of Western Australia, Perth, WA
- 10 Royal Melbourne Hospital, Melbourne, VIC
- 11 Canberra Hospital, Canberra, ACT
- 12 Westmead Private Hospital, Sydney, NSW
- 13 University of Melbourne, Melbourne, VIC
- 14 Western Sydney University, Sydney, NSW
- 15 Consumer Representative, Perth, WA
- 16 National Heart Foundation of Australia, Melbourne, VIC
A full conflict of interest register is available at:
https://www.heartfoundation.org.au/for-professionals/clinical-information/heart-failure
- 1. National Heart Foundation of Australia, Cardiac Society of Australia and New Zealand (Chronic Heart Failure Guidelines Expert Writing Panel). Guidelines for the prevention, detection and management of chronic heart failure in Australia. Updated 2011. Melbourne: National Heart Foundation of Australia, 2011. https://www.heartfoundation.org.au/images/uploads/publications/Chronic_Heart_Failure_Guidelines_2011.pdf (viewed July 2018).
- 2. Atherton J, Sindone A, De Pasquale C, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart Lung Circ 2018; https://doi.org/10.1016/j.hlc.2018.06.1042.
- 3. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (viewed Feb 2018).
- 4. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387: 957-967.
- 5. Preiss D, Campbell RT, Murray HM, et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J 2015; 36: 1536-1546.
- 6. Zhang XL, Zhu QQ, Chen YH, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc 2018; 7: e007165.
- 7. The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327: 685-691.
- 8. Doust JA, Glasziou PP, Pietrzak E, et al. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med 2004; 164: 1978-1984.
- 9. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277-314.
- 10. Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000; 355: 1575-1581.
- 11. CIBIS Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomized trial. Lancet 1999; 353: 9-13.
- 12. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999; 353: 2001-2007.
- 13. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651-1658.
- 14. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26: 215-225.
- 15. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709-717.
- 16. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11-21.
- 17. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362: 772-776.
- 18. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993-1004.
- 19. Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376: 875-885.
- 20. Faris RF, Flather M, Purcell H, et al. Diuretics for heart failure. Cochrane Database Syst Rev 2012; (2): CD003838.
- 21. Hopper I, Samuel R, Hayward C, et al. Can medications be safely withdrawn in patients with stable chronic heart failure? systematic review and meta-analysis. J Card Fail 2014; 20: 522-532.
- 22. Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; doi: 10.1002/ejhf.1149
- 23. Cleland JGF, Bunting KV, Flather MD, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018; 39: 26-35.
- 24. Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455-462.
- 25. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383-1392.
- 26. McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810-819.
- 27. Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015; (10): CD007228.
- 28. Driscoll A, Currey J, Tonkin A, et al. Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction. Cochrane Database Syst Rev 2015; (12): CD009889.
- 29. Sagar VA, Davies EJ, Briscoe S, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015; 2: e000163.
- 30. Cleland JG, Abraham WT, Linde C, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013; 34: 3547-3556.
- 31. Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart 2015; 101: 1800-1806.
- 32. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013; 368: 1585-1593.
- 33. Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013; 369: 1395-1405.
- 34. Kutyifa V, Stockburger M, Daubert JP, et al. PR interval identifies clinical response in patients with non-left bundle branch block: a Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ Arrhythm Electrophysiol 2014; 7: 645-651.
- 35. Koplan BA, Kaplan AJ, Weiner S, et al. Heart failure decompensation and all cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol 2009; 53: 355-360.
- 36. Moss AJ, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877-883.
- 37. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225-237.
- 38. Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221-1230.
- 39. Stavrakis S, Asad Z, Reynolds D. Implantable cardioverter defibrillators for primary prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. J Cardiovasc Electrophysiol 2017; 28: 659-665.
- 40. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016; 374: 1511-1520.
- 41. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739-2791.
- 42. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597-1607.
- 43. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187-2198.
- 44. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790-1798.
- 45. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609-1620.
- 46. Mehra MR, Goldstein DJ, Uriel N, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018; 378: 1386-1395.
- 47. Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016; 35: 1158-1169.
- 48. Li SJ, Sartipy U, Lund LH, et al. Prognostic significance of resting heart rate and use of beta-blockers in atrial fibrillation and sinus rhythm in patients with heart failure and reduced ejection fraction: findings from the Swedish Heart Failure Registry. Circ Heart Fail 2015; 8: 871-879.
- 49. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of atrial fibrillation 2018. Med J Aust 2018; https://doi.org/10.5694/mja18.00646 [Epub ahead of print].
- 50. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018; 378: 417-427.
- 51. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373: 1095-1105.
- 52. Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368: 1210-1219.
- 53. Jankowska EA, Tkaczyszyn M, Suchocki T, et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786-795.
- 54. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016; 316: 2104-2114.





Abstract
Introduction: Heart failure (HF) is a clinical syndrome that is secondary to an abnormality of cardiac structure or function. These clinical practice guidelines focus on the diagnosis and management of HF with recommendations that have been graded on the strength of evidence and the likely absolute benefit versus harm. Additional considerations are presented as practice points.
Main recommendations:
Changes in management as a result of the guideline: These guidelines have been designed to facilitate the systematic integration of recommendations into HF care. This should include ongoing audit and feedback systems integrated into work practices in order to improve the quality of care and outcomes of patients with HF.