The known Community support for medical cannabis is growing in Australia, but patterns of use and consumer views are poorly understood.
The new Participants predominantly used medical cannabis for chronic pain, mental health, sleep problems, and neurological conditions. It was typically procured illegally and inhaled. Problems associated with illicit supply included variable product quality, cost, access, and legal and employment worries. Most participants reported positive health outcomes, but also frequent side effects.
The implications Consumer perspectives of the role of medical cannabis and preferred models of its availability differ from those of professional medical groups.
In response to consumer demand and with widespread community support, recent legislative changes1,2 have made cannabis products legally available in Australia within Therapeutic Goods Administration frameworks for the use of unlicensed medicines, including the Special Access and Authorised Prescriber schemes.3 However, the uptake of prescribed medical cannabis has been limited amid caution from medical professional groups.4,5
Community support for prescribed medical cannabis continues to grow,6 possibly influenced by media reporting; surveys of patients with chronic pain,7 cancer,8 and epilepsy9 have found that considerable numbers of Australians have either used (10–20%) or would consider using cannabis products for therapeutic purposes. However, knowledge of the unregulated use of medical cannabis in Australian remains imprecise because it is generally illegal.
To better understand the use of medical cannabis in Australia immediately prior to the legislation in 2016 of frameworks for its use, we examined the characteristics of people who employed cannabis for self-identified medical reasons, the range of conditions or symptoms for which cannabis is used, patterns of use, perceived benefits and harms, and consumer perspectives on how medical cannabis should be regulated. Given that most medical cannabis use in Australia is illegal, an anonymous online survey was the most appropriate approach.
“Medical cannabis” in this article reflects the meaning generally understood by laypersons; we do not imply its use has been authorised or prescribed by a medical practitioner, nor that there is evidence for its efficacy in treating particular conditions.
Methods
We administered a cross-sectional online survey to a convenience sample of individuals who reported that they had used cannabis for therapeutic reasons during the preceding 12 months. Eligible to participate were adults (aged 18 years or more) who provided informed consent and reported using cannabis or a cannabinoid product for a medical purpose in the preceding 12 months.
The survey questions were developed by the investigators, building upon previous consumer surveys10-13 and incorporating questions relevant to current Australian conditions. Areas of enquiry included:
-
participant characteristics;
-
medical conditions and symptoms for which medical cannabis was used, in structured checklists of conditions and symptoms for which medical cannabis use has previously been reported;
-
current and lifetime patterns of cannabis use, including preparation type and source, frequency (days in past month, average number times per day) and route of use, and cost;
-
perceived benefits and harms associated with medical cannabis, including side effects (symptom checklist), social and legal implications, Patient Global Impression of Change14 (a validated 7-item self-assessment of improvement or deterioration in symptoms), cannabis use disorder in the past 12 months as assessed with the 11 DSM-5 criteria15 (2–3 criteria met, mild; 4–5, moderate; 6 or more, severe), and cannabis withdrawal syndrome15 (three or more of seven symptoms on stopping regular cannabis use);
-
consumer perspectives of how medical cannabis should be available in Australia.
The survey was refined after piloting with consumers, clinicians and researchers; data were then collected during April–October 2016. The survey was promoted in online media and on consumer group webpages, including Facebook groups, and at professional and medical cannabis consumer forums. Data were collected and managed with Research Electronic Data Capture (REDcap), a secure web-based platform that allows participants to directly enter their responses online.
Data analysis
Descriptive statistics were calculated in SPSS 24 (IBM). The levels of missing data were high (up to 40% for some questions), particularly toward the end of the questionnaire; rather than imputing data, valid responses only are reported.
Ethics approval
This study was approved by the University of Sydney Human Research Ethics Committee (reference, 2015/234).
Results
Participants
Of 2281 participants who logged into the survey, 152 were ineligible (no informed consent, 146; under 18 years, six). Data from a further 381 respondents were excluded because they completed only consent and eligibility survey items. The final sample therefore included 1748 participants.
Most participants were recruited through Facebook groups (1437 participants); other sources were friends (66), online forums (65), the Lambert Initiative website (35), medical cannabis providers (20), consumer support groups (18), Twitter (13), health care providers (11), and other (153). Participants reported their main residence as being in New South Wales or the Australian Capital Territory (602 of 1724 respondents, 34.9%), Queensland (494, 28.7%), Victoria (277, 16.1%), Western Australia (172, 10.0%), South Australia (115, 6.7%), and Tasmania (64, 3.7%).
The demographic characteristics of the sample are summarised in Box 1. Most respondents were men (68.1%); their mean age was 37.9 years (standard deviation [SD], 13.4 years), 56.6% were either engaged in paid employment or home duties, and 21.5% received disability pensions.
Cannabis use
Participants had used cannabis for medical reasons for a mean of 9.8 years (SD, 12.5 years). About half (642 of 1161 respondents, 55.3%) reported having used cannabis for recreational reasons at the time they commenced medical cannabis use, while 355 (30.6%) reported prior recreational use but not immediately before commencing medical cannabis (mean gap, 6.6 years; SD, 8.3 years; median gap, 3 years); 164 (14.1%) had not used cannabis before using it for medical reasons.
Most participants reported that their cannabis use was mainly for medical reasons (mean for 1421 responses, 81% of use; SD, 20%); respondents used medical cannabis a median three times per day on a mean of 19.9 (SD, 10 days) of the preceding 28 days (median, 26 of preceding 28 days; 1443 responses). One-quarter of participants (27.5% of 1253 respondents) did not routinely pay for medical cannabis; the 909 respondents who did pay for it spent a mean $94.50 (SD, $86.60) per week (Box 2). The most frequent routes of administration were by inhalation (83.4%) (Box 3).
About half the respondents (659 of 1328, 49.6%) reported stopping medical cannabis at least temporarily during the past 12 months. Reasons included problems with supply (433, 65.7%), expense (274, 41.6%), legal aspects (including work-related drug testing: 87, 6.6%), no longer having the treated complaint (38, 5.8%), concerns about tolerance or addiction (19, 2.9%), not enjoying the psychoactive aspect (19, 2.9%), not liking the side effects (16, 2.4%), lack of effect (2, 0.3%), and other (43, 6.5%).
Indications for using medical cannabis and patient-reported outcomes
When asked to identify from a structured list up to five medical conditions for which they were using medical cannabis, the conditions most frequently reported by 1629 respondents were anxiety (50.7%), back pain (50.0%), depression (49.3%), sleep problems (43.5%), neck pain (25.6%), and post-traumatic stress disorder (22.9%). When asked to identify the main condition for which they used medical cannabis, the most frequently cited conditions were back pain (15.2%), anxiety (14.9%) and depression (10.9%); by category, 593 respondents (36.4%) nominated pain conditions, 591 (36.3%) mental health conditions, 122 (7.5%) neurological conditions, 108 (6.6%) sleep conditions, and 215 (13.2%) other problems (Box 4).
The specific symptoms most frequently managed with medical cannabis were pain (69.4% of respondents), sleep abnormalities (69.2%), and anxiety (57.5%). Large majorities of participants (71–92%) indicated that the treated symptom had very much or much improved; apart from appetite/weight control (1.6%) and anxiety (1.0%), fewer than 1% of respondents felt that the treated symptom had worsened (Box 5).
Side effects and other adverse consequences
The side effects most frequently selected from a structured checklist by 1302 participants were increased appetite (74.0%), drowsiness (67.1%), ocular irritation (40.7%), lack of energy (37.5%), memory impairment (31.6%), palpitations (15.4%), and paranoia (15.2%) or confusion (12.4%). Severe or intolerable side effects were infrequent (fewer than 2% of respondents, except for increased appetite, 6.1%) (Box 6).
DSM-5 criteria for cannabis use disorder were met by 456 of 1118 respondents (40.8%): 267 (23.9%) for mild, 118 (10.6%) for moderate, and 71 (6.4%) for severe cannabis use disorder. DSM-5 criteria for cannabis withdrawal syndrome were met by 387 of 1118 respondents (43.3%), with sleep problems the most frequent symptom (61.7%).
Thirty-five of 1092 respondents (3.2%) reported having been convicted for driving while under the influence of cannabis, 927 (84.9%) reported being worried about being arrested or other legal problems, and 518 (47.4%) were concerned about employment security because of medical cannabis use.
Procuring medical cannabis
The main sources of medical cannabis were recreational cannabis dealers (578 of 1255 responses, 46.1%), friends or family (407, 32.4%), own plants (142, 11.3%), medical cannabis suppliers (62, 4.9%), online suppliers (28, 2.2%), overseas suppliers (four, 0.3%), clubs or cooperatives (four, 0.3%), a pharmacy (on prescription from a medical professional: one, 0.1%), and other (29, 2.3%).
A total of 674 of 1254 respondents (53.7%) reported they had been unable to access medical cannabis at some time during the preceding 28 days. Asked to rate the extent to which they worried about access to their preferred source, 950 of 1256 respondents reported some concern (very concerned, 46.7%; somewhat concerned, 28.9%); 19.9% had few or no concerns, and 4.5% offered no opinion. About half (653 of 1255 respondents, 52.0%) indicated that the quality of their medical cannabis was not consistent over time. The cost of medical cannabis placed a significant strain on finances for 614 of 1092 respondents (56.2%).
A total of 418 of 1142 respondents (36.6%) had not discussed their medical cannabis use with health care providers, 311 (27.2%) discussed it with a small number of their health care providers, and 413 (36.2%) with most of their health care providers. Of the 724 respondents who had discussed their use, the most frequently consulted health care providers were general practitioners (84.1%), specialist medical practitioners (61.6%), counsellors (36.6%), nurses (24.3%), pharmacists (18.2%), and alternative health practitioners (17.8%).
Consumer perspectives on future treatment models
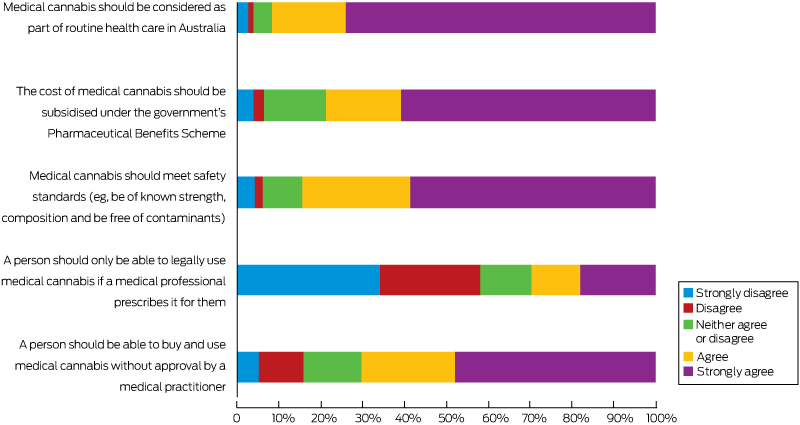
Most respondents (960 of 1081, 88.8%) believed that cannabis use should be legal for all purposes (recreational and medical), 119 (11.0%) that it should be legal only for medical reasons, and two participants (0.2%) that all use should remain illegal. Participants were willing to pay a mean $11.00 (SD, $9.80) per day for medical cannabis. Further responses are summarised in Box 7.
Discussion
This is the first Australian survey of people using cannabis for self-identified medical reasons in more than a decade.13 As the survey was conducted immediately before Australian regulatory changes in November 2016, it is not surprising that almost all participants reported using illicit preparations. Like other international surveys,10-12 we found that medical cannabis is used for a diverse range of health conditions, especially pain, mental health, sleep, and neurological conditions. More than 80% of respondents indicated that medical cannabis effectively managed their target symptom. This may reflect both placebo effects (likely for a psychoactive, consumer-selected product) and sampling bias, and it is notable that the reported positive outcomes contrast with findings from clinical trials and other investigations of the efficacy of cannabinoids for many of these conditions.16,17 While certain cannabis products are reported to be effective for some patients with pain, sleep problems, chemotherapy-induced nausea and vomiting, or spasticity in multiple sclerosis,16,17 clinical trial evidence regarding many of the conditions cited by our respondents is limited, and cannabis may even exacerbate symptoms such as anxiety or psychosis in some patients.
Most of our respondents, and indeed most of the broader Australian community,6 believe cannabis can play a role in medicine, but peak medical groups are cautious in the absence of a robust evidence base for guiding clinical practice.4,5 This dissonance between consumer and professional groups colours discussions about the future availability of medical cannabis in Australia; 92% of our respondents believe that medical cannabis should be integrated into mainstream health care, and 79% that it be subsidised by the government; only 30% agreed that it should be available only on prescription. Most participants were willing to pay $10–$15 per day for medical cannabis, as they do now. However, unlicensed medicines are not eligible for Pharmaceutical Benefits Scheme subsidies, so the cost of prescribed medical cannabis may be a barrier for many. Two-thirds of respondents had discussed medical cannabis with health practitioners, usually general practitioners or medical specialists, underlining the need to educate the medical community,4 particularly about potential benefits and harms and the medico-legal frameworks for medical cannabis treatment.
There was a widespread belief among respondents that medical cannabis products should meet safety standards, be of consistent strength and composition, and be free of contaminants. Reliance on illicit supplies exposed most respondents to products of inconsistent quality and uncertain availability, and also caused legal and employment concerns. Nearly 60% of respondents would prefer routes of administration they perceive to be safer than smoking, including oral, topical, suppository, and vaporiser approaches, currently used by only 29.4% of respondents.
A range of other safety problems were identified, including common side effects that could have clinical implications, such as drowsiness, fatigue, memory impairment, cardiac symptoms (eg, palpitations), paranoia, anxiety, hallucinations, and confusion. While these effects have previously been reported,18 their higher prevalence among our respondents than in clinical trials may be explained by our data collection method (self-report from a symptom checklist), higher dosages among self-medicators, or administration by smoking.
Seventeen per cent of respondents met DSM-5 criteria for moderate or severe cannabis use disorder, so-called “cannabis dependence”, similar to the rate in an Australian study of patients using illicit medical cannabis to manage pain.7 Clinical models of treatment should consider how dependence is managed in patients taking medical cannabis for longer periods; a universal precautions framework, as recommended for opioid use in chronic pain management,19 may be appropriate.
Limitations
Anonymous and convenience sampling enabled us to reach hidden populations engaged in illicit behaviours, but introduced sampling bias, in that only those individuals with a minimum degree of literacy and access to the internet, and actively using medical cannabis were eligible to participate; dissatisfied individuals no longer using medical cannabis (because of side effects or poor efficacy, for example) were excluded. The degree of recruitment from online medical cannabis consumer forums also introduces sampling bias; for example, the rate of lifetime cannabis use (prior to medical cannabis use) was much higher in our survey than in the general Australian population6 or a large cohort of Australian patients with chronic pain.7 Nevertheless, our sample was comparable with these patients7 with respect to demographic characteristics, age at initiation and duration of medical cannabis use, and the high rates of perceived efficacy, suggesting our findings could be generalised to other patients using illicit medical cannabis.
The inability to corroborate responses with data from independent sources is another limitation. Our study relied on self-reporting, including the perception that cannabis was being used for a medical reason. Community debate about cannabis regulations may have encouraged some respondents to provide responses that favoured legalisation of medical cannabis. There were high rates of missing data for some questions, particularly toward the end of the survey, probably reflecting responder fatigue. The study design did not enable us to estimate the prevalence of medical cannabis use in the community. Nevertheless, our survey provides valuable insights into unregulated medical cannabis use in Australia at a time of marked policy and clinical change.
Conclusion
Illicitly obtained cannabis is used by consumers for managing a variety of health conditions in Australia, including conditions for which there is limited formal evidence of safety and efficacy. As consumer demand for medical cannabis increases, it is important that health providers better understand the reasons for and the patterns of medical cannabis use that will influence requests from their patients. It is also important that health providers understand that their patients’ experiences of medical cannabis may not accord with reported clinical findings. While most respondents reported health benefits, side effects were common, and there were also several undesirable consequences of relying on illicit cannabis supplies, including variable product quality, expense, difficult access, and legal and employment problems. It remains to be seen how the new legal regulations for prescribed medical cannabis will translate into clinical practice in Australia, and whether consumer demand can be met within an appropriate evidence and safety framework.
Box 1 – Demographic characteristics of the 1748 eligible respondents to the Cannabis as Medicine Survey
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Age when completing survey (years), mean (SD) |
37.9 (13.4) |
||||||||||||||
|
Sex |
|
||||||||||||||
|
Women |
545 (31.2%) |
||||||||||||||
|
Men |
1190 (68.1%) |
||||||||||||||
|
Other |
13 (0.7%) |
||||||||||||||
|
Current relationship status |
|
||||||||||||||
|
Single and never married |
643 (36.8%) |
||||||||||||||
|
Married/domestic partnership |
757 (43.3%) |
||||||||||||||
|
Divorced/separated |
297 (17.0%) |
||||||||||||||
|
Widowed |
28 (1.6%) |
||||||||||||||
|
Missing data |
23 (1.3%) |
||||||||||||||
|
Indigenous status |
|
||||||||||||||
|
Aboriginal and/or Torres Strait Islander |
95 (5.4%) |
||||||||||||||
|
Not Aboriginal and/or Torres Strait Islander |
1653 (94.6%) |
||||||||||||||
|
Highest education level |
|
||||||||||||||
|
Primary school |
20 (1.1%) |
||||||||||||||
|
Secondary/high school |
652 (37.3%) |
||||||||||||||
|
Trade or vocational training |
672 (38.4%) |
||||||||||||||
|
University degree |
348 (19.9%) |
||||||||||||||
|
Other |
56 (3.2%) |
||||||||||||||
|
Current employment status |
|
||||||||||||||
|
Full-time work |
559 (32.0%) |
||||||||||||||
|
Casual or part-time work |
316 (18.1%) |
||||||||||||||
|
Home duties |
113 (6.5%) |
||||||||||||||
|
Student |
102 (5.8%) |
||||||||||||||
|
Unemployed |
116 (6.6%) |
||||||||||||||
|
Disability pension |
376 (21.5%) |
||||||||||||||
|
Retired |
63 (3.6%) |
||||||||||||||
|
Other |
103 (5.9%) |
||||||||||||||
|
|
|||||||||||||||
|
SD = standard deviation. |
|||||||||||||||
Box 2 – Cannabis use reported by the 1748 eligible respondents to the Cannabis as Medicine Survey
|
Characteristic |
Responses |
|
|||||||||||||
|
|
|||||||||||||||
|
Age, first used cannabis (any reason) (years), mean (SD) |
1748 |
18.6 (8.9) |
|||||||||||||
|
Age, first regular cannabis use (any reason) (years), mean (SD) |
1748 |
23.7 (11.0) |
|||||||||||||
|
Age, first used cannabis for medical reason (years), mean (SD) |
1446 |
27.4 (13.0) |
|||||||||||||
|
Age, first regular cannabis use for medical reason (years), mean (SD) |
1748 |
29.2 (12.8) |
|||||||||||||
|
Used cannabis in past 28 days (any reason) (days) |
1429 |
|
|||||||||||||
|
Mean (SD) |
|
20.9 (9.4) |
|||||||||||||
|
Median (IQR) |
|
26 (15–28) |
|||||||||||||
|
Used cannabis in past 28 days (medical reasons) (days) |
1443 |
|
|||||||||||||
|
Mean (SD) |
|
19.9 (10.0) |
|||||||||||||
|
Median (IQR) |
|
26 (12–28) |
|||||||||||||
|
Estimated proportion of cannabis use for medical reasons |
1421 |
|
|||||||||||||
|
Mean (SD) |
|
81% (20%) |
|||||||||||||
|
Median (IQR) |
|
87% (71–98%) |
|||||||||||||
|
Usual number of times cannabis used per day (any reason) |
1140 |
|
|||||||||||||
|
Mean (SD) |
|
5.0 (4.9) |
|||||||||||||
|
Median (IQR) |
|
3 (2–6) |
|||||||||||||
|
Weekly cost of medical cannabis |
1253 |
|
|||||||||||||
|
Mean (SD) |
|
$68.60 ($85.00) |
|||||||||||||
|
Median (IQR) |
|
$50 (0–$100) |
|||||||||||||
|
Weekly cost of medical cannabis (excluding respondents who did not pay for cannabis) |
909 |
|
|||||||||||||
|
Mean (SD) |
|
$94.50 ($86.60) |
|||||||||||||
|
Median (IQR) |
|
$75 ($40–$120) |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 3 – Routes of administration of medical cannabis during the preceding 12 months (1450 respondents) and preferred route were medical cannabis legally available (1081 respondents)
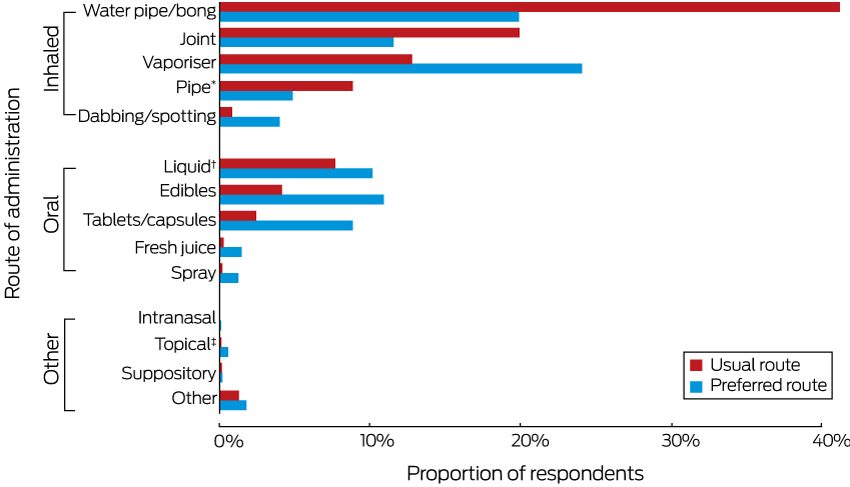
* Including plastic, metal, glass. † Including oils, tinctures. ‡ Including creams, patches.
Box 4 – Conditions reported as reasons for using medical cannabis by 1629 respondents
|
Condition |
Reasons for using medicinal cannabis |
||||||||||||||
|
All reasons∗ |
Main reason† |
||||||||||||||
|
|
|||||||||||||||
|
Mental health |
|
591 (36.3%) |
|||||||||||||
|
Anxiety disorder (eg, generalised anxiety, panic disorder, obsessive–compulsive disorder) |
826 (50.7%) |
242 (14.9%) |
|||||||||||||
|
Depression |
802 (49.3%) |
177 (10.9%) |
|||||||||||||
|
Post-traumatic stress disorder |
373 (22.9%) |
86 (5.3%) |
|||||||||||||
|
Attention deficit disorder |
181 (11.1%) |
43 (2.6%) |
|||||||||||||
|
Schizophrenia or other psychosis |
50 (3.1%) |
21 (1.3%) |
|||||||||||||
|
Addiction (alcohol, opioid, amphetamine) |
128 (7.9%) |
14 (0.9%) |
|||||||||||||
|
Addiction (cannabis) |
131 (8.1%) |
0 |
|||||||||||||
|
Eating disorders |
152 (9.3%) |
8 (0.5%) |
|||||||||||||
|
Pain |
|
593 (36.4%) |
|||||||||||||
|
Back pain |
813 (50.0%) |
248 (15.2%) |
|||||||||||||
|
Neck pain |
417 (25.6%) |
31 (1.9%) |
|||||||||||||
|
Arthritis (eg, rheumatoid, osteoarthritis) |
309 (19.0%) |
105 (6.5%) |
|||||||||||||
|
Neuropathy |
286 (17.6%) |
80 (4.9%) |
|||||||||||||
|
Migraines or regular headaches |
340 (20.9%) |
44 (2.7%) |
|||||||||||||
|
Fibromyalgia |
102 (6.3%) |
38 (2.3%) |
|||||||||||||
|
Spinal cord injury |
118 (7.3%) |
36 (2.2%) |
|||||||||||||
|
Gynaecological pain |
16 (1.0%) |
11 (0.7%) |
|||||||||||||
|
Sleep condition (eg, insomnia, sleep apnoea) |
707 (43.5%) |
108 (6.6%) |
|||||||||||||
|
Neurological |
|
122 (7.5%) |
|||||||||||||
|
Epilepsy or seizure disorder |
127 (7.8%) |
78 (4.8%) |
|||||||||||||
|
Multiple sclerosis |
20 (1.2%) |
19 (1.2%) |
|||||||||||||
|
Autism |
38 (2.3%) |
12 (0.7%) |
|||||||||||||
|
Dementia |
5 (0.3%) |
5 (0.3%) |
|||||||||||||
|
Parkinson disease |
7 (0.4%) |
3 (0.2%) |
|||||||||||||
|
Tourette syndrome |
3 (0.2%) |
3 (0.2%) |
|||||||||||||
|
Amyotrophic lateral sclerosis |
2 (0.1%) |
0 |
|||||||||||||
|
Glaucoma |
23 (1.4%) |
2 (0.1%) |
|||||||||||||
|
Huntington disease |
1 (< 0.1%) |
0 |
|||||||||||||
|
Miscellaneous |
|
215 (13.2%) |
|||||||||||||
|
Gastrointestinal conditions (eg, irritable bowel syndrome, Crohn disease, ulcerative colitis) |
202 (12.4%) |
60 (3.7%) |
|||||||||||||
|
Cancer |
80 (4.9%) |
58 (3.6%) |
|||||||||||||
|
Auto-immune conditions (eg systemic lupus erythematosus, chronic fatigue disorder) |
107 (6.6%) |
32 (2.0%) |
|||||||||||||
|
Respiratory (eg, asthma, cystic fibrosis) |
164 (10.1%) |
23 (1.4%) |
|||||||||||||
|
Infectious diseases (e.g. AIDS/HIV, viral hepatitis) |
26 (1.6%) |
12 (0.7%) |
|||||||||||||
|
Cardiovascular disease/diabetes |
23 (1.4%) |
11 (0.7%) |
|||||||||||||
|
Skin condition (eg, eczema, psoriasis, dermatitis) |
153 (9.4%) |
8 (0.5%) |
|||||||||||||
|
Other |
251 (15.4%) |
11 (0.7%) |
|||||||||||||
|
|
|||||||||||||||
|
*Participants asked to identify up to five conditions for which they had used medicinal cannabis in the past 12 months. †Participants asked to report the condition they identified as the main reason for using medicinal cannabis in the past 12 months. |
|||||||||||||||
Box 5 – Symptoms managed by 1588 respondents with medical cannabis*
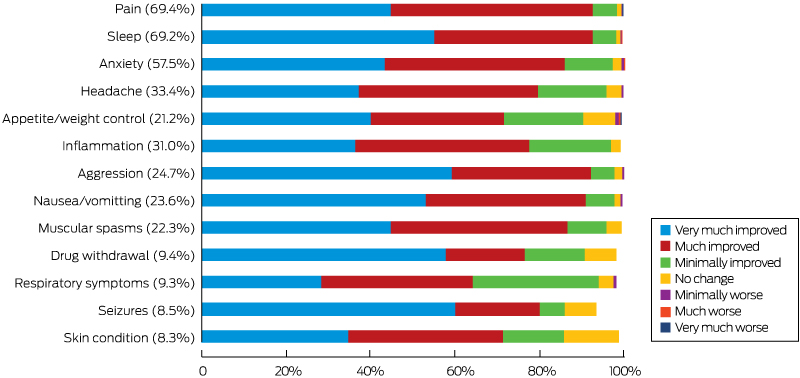
* The proportion of respondents using medical cannabis for each symptom is indicated in parentheses. For each symptom, a small number (fewer than 0–7% of respondents for each symptom) did not provide Patient Global Impression of Change ratings, so that responses do not sum to 100%.
Box 6 – Side effect profile of medical cannabis use by 1302 respondents
|
|
Side effect |
Severe or intolerable side effect |
|||||||||||||
|
|
|||||||||||||||
|
Increased appetite |
963 (74.0%) |
79 (6.1%) |
|||||||||||||
|
Drowsiness |
873 (67.1%) |
23 (1.8%) |
|||||||||||||
|
Ocular irritation (red, sore, itchy eyes) |
530 (40.7%) |
13 (1.0%) |
|||||||||||||
|
Lack of energy |
488 (37.5%) |
16 (1.2%) |
|||||||||||||
|
Memory impairment |
412 (31.6%) |
7 (0.5%) |
|||||||||||||
|
Racing heart (palpitations) |
201 (15.4%) |
2 (0.2%) |
|||||||||||||
|
Paranoia |
198 (15.2%) |
14 (1.1%) |
|||||||||||||
|
Decreased appetite |
171 (13.1%) |
5 (0.4%) |
|||||||||||||
|
Confusion |
162 (12.4%) |
3 (0.2%) |
|||||||||||||
|
Dizziness |
124 (9.5%) |
5 (0.4%) |
|||||||||||||
|
Anxiety |
119 (9.1%) |
9 (0.7%) |
|||||||||||||
|
Sweating |
95 (7.3%) |
3 (0.2%) |
|||||||||||||
|
Difficulty controlling movements |
78 (6.0%) |
2 (0.1%) |
|||||||||||||
|
Gastrointestinal irritation |
62 (4.8%) |
4 (0.3%) |
|||||||||||||
|
Headache |
57 (4.4%) |
4 (0.3%) |
|||||||||||||
|
Indigestion |
55 (4.2%) |
1 (0.3%) |
|||||||||||||
|
Depressed mood |
52 (4.0%) |
5 (0.4%) |
|||||||||||||
|
Diarrhoea |
52 (4.0%) |
4 (0.3%) |
|||||||||||||
|
Hallucinations |
38 (2.9%) |
0 |
|||||||||||||
|
Shaking/tremor |
37 (2.8%) |
5 (0.4%) |
|||||||||||||
|
Constipation |
29 (2.2%) |
1 (0.1%) |
|||||||||||||
|
Delusions |
18 (1.4%) |
1 (0.1%) |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 15 December 2017, accepted 1 March 2018
- Nicholas Lintzeris1,2
- Jessica Driels3
- Natalie Elias4
- Jonathon C Arnold1,4
- Iain S McGregor1,4
- David J Allsop4
- 1 University of Sydney, Sydney, NSW
- 2 South Eastern Sydney Local Health District, Sydney, NSW
- 3 Fulham Correctional Centre, West Sale, VIC
- 4 Lambert Initiative for Cannabinoid Therapeutics, University of Sydney, Sydney, NSW
This study was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded centre for medicinal cannabis research at the University of Sydney and the South Eastern Sydney Local Health District, NSW Health. Nicholas Lintzeris has received funding for investigator-led research, and has sat on an advisory board for Indivior (buprenorphine products; unrelated to this investigation). He has been reimbursed for participating in a research trial of a depot buprenorphine medication sponsored by Braeburn–Camurus (unrelated to this investigation) and acted as a consultant and sat on an advisory board for Mundipharma (naloxone product; unrelated to this investigation). Iain McGregor is Academic Director of the Lambert Initiative and a National Health and Medical Research Council (NHMRC) Principal Research Fellow; he receives funding from the Australian Research Council and the NHMRC, and is involved in an NHMRC-funded clinical trial of the cannabis extract, Sativex.
- 1. Advisory Committee on Medicine Scheduling. Final decisions and reasons for decisions by a delegate of the Secretary to the Department of Health [report]. Australian Government Department of Health, Therapeutic Goods Administration; 31 Aug 2016. https://www.tga.gov.au/sites/default/files/scheduling-delegates-final-decisions-cannabis-and-tetrahydrocannabinols-march-2016_0.pdf (viewed Nov 2017).
- 2. Australian Government. Narcotic Drugs Amendment Act 2016, No.12, 2016. Federal Register of Legislation; 2 Mar 2016. https://www.legislation.gov.au/Details/C2016A00012 (viewed Nov 2017).
- 3. McEwen J. A history of therapeutic goods regulation in Australia. Canberra: Commonwealth of Australia, 2007. https://www.tga.gov.au/sites/default/files/history-tg-regulation.pdf (viewed Nov 2017).
- 4. Royal Australian College of Physicians (RACGP). RACGP position statement: medicinal use of cannabis products. Oct 2016. https://www.racgp.org.au/support/policies/clinical-and-practice-management/racgp-position-statement-medicinal-use-of-cannabis-products/ (viewed Nov 2017).
- 5. Johnson C. Green light for medicinal cannabis but AMA says proceed with caution [internet]. Australian Medicine (Australian Medical Association), 3 Mar 2017. https://ama.com.au/ausmed/green-light-medicinal-cannabis-ama-says-proceed-caution (viewed Nov 2017).
- 6. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2016: detailed findings (AIHW Cat. No. PHE 214; Drug Statistics Series No. 31). Canberra: AIHW, 2017.
- 7. Degenhardt L, Lintzeris N, Campbell G, et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 2015; 147: 144-150.
- 8. Luckett T, Phillips J, Lintzeris N, et al. Clinical trials of medicinal cannabis for appetite-related symptoms from advanced cancer: a survey of preferences, attitudes and beliefs among patients willing to consider participation. Intern Med J 2016; 46: 1269-1275.
- 9. Suraev AS, Todd L, Bowen MT, et al. An Australian nationwide survey on medicinal cannabis use for epilepsy: history of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav 2017; 70(Pt B): 334-340.
- 10. Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy 2013; 24: 511-516.
- 11. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids — an international cross-sectional survey on administration forms. J Psychoactive Drugs 2013; 45: 199-210.
- 12. Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res 2016; 1: 131-138.
- 13. Swift W, Gates P, Dillon P. Survey of Australians using cannabis for medical purposes. Harm Reduct J 2005; 2: 18.
- 14. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004; 27: 26-35.
- 15. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fifth edition (DSM-5). Arlington (VA): American Psychiatric Association, 2013.
- 16. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313: 2456-2473.
- 17. National Academies of Sciences, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington: National Academies Press, 2017. https://www.nap.edu/catalog/24625/the-health-effects-of-cannabis-and-cannabinoids-the-current-state (viewed Feb 2018).
- 18. Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013; 33: 195-209.
- 19. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6: 107-112.





Abstract
Objective: To explore patterns of cannabis use for medical purposes in Australia immediately prior to the 2016 legislation for frameworks for medical cannabis use.
Design, setting: Anonymous online survey with convenience sample, April–October 2016. Participants were recruited through online media and at professional and consumer forums.
Participants: Adults (at least 18 years of age) who reported using a cannabis product for self-identified medical or therapeutic reasons during the preceding 12 months.
Main outcome measures: Consumer characteristics; indications and patterns of medical cannabis use; perceived benefits and harms; views on appropriate availability of medical cannabis.
Results: Most of the 1748 participants were men (68.1%) and employed (56.6%), with a mean age of 37.9 years (SD, 13.4 years) and mean reported period of medical cannabis use of 9.8 years (SD, 12.5 years). The most frequent reasons for medical cannabis use were anxiety (50.7%), back pain (50.0%), depression (49.3%), and sleep problems (43.5%). Respondents had used medical cannabis on a mean of 19.9 of the previous 28 days (SD, 10.0 days), spending a mean $68.60 (SD, $85.00) per week, and 83.4% had inhaled the substance. Participants reported high levels of clinical effectiveness and frequent side effects, including drowsiness, ocular irritation, lethargy and memory impairment; 17% met DSM-5 criteria for moderate or severe cannabis use disorder. Many reported harms or concerns related to the illicit status of cannabis. Participants believed that medical cannabis should be integrated into mainstream health care, and that products should be required to meet consistency and safety standards.
Conclusion: Illicitly sourced cannabis is used to treat a broad range of medical conditions in Australia. Future models of prescribed medical cannabis take consumer patterns of use and demand into consideration.