Comorbidity is the presence of one or more diseases or disorders occurring concurrently with a primary disease or disorder. In asthma, comorbid conditions are frequently present, and they add to the burden of respiratory symptoms associated with the primary condition. They often contribute to a severe and difficult-to-treat asthma phenotype.1
The link between comorbidity and asthma is complex. Some comorbid conditions can both occur together with asthma and masquerade as asthma itself, as with vocal cord dysfunction (VCD). Other conditions or factors can affect the nature and severity of asthma, leading to a distinct phenotype. Teasing apart the different components of comorbidity in severe asthma can be difficult, and general practitioners are often confronted by complexity that may seem beyond the scope of everyday care.
As asthma care has increasingly focused on personalised management for severe asthma, recognition of the role and importance of comorbid conditions has increased. To improve overall management and care, doctors need to take these concurrent disorders into account, and one way to simplify this process is to consider comorbid conditions as a “bundle” of disease traits that may be amendable to treatment — so-called “treatable traits”.2 Managing these traits can potentially improve asthma control, reduce or remove the need for some medications and improve overall quality of life for many patients considered to have severe asthma.3 Additionally, the appropriate treatment of comorbid conditions is a critical step in distinguishing the subset of patients with biologically severe asthma from among the undifferentiated group of patients presenting with difficult-to-treat asthma.4 This has important implications for therapy, as outlined elsewhere in this supplement in the article on the diagnosis of severe asthma.5
In this review, we provide a brief overview of key comorbid conditions that are likely to be encountered in general practice. We also outline a simplified, iterative approach to assessing and managing comorbid conditions in severe asthma.
In conducting this review, we searched English language literature in PubMed using the key words severe asthma, comorbidity, treatment and management.
The spectrum of comorbid conditions in severe asthma
A broad spectrum of comorbid conditions has been noted in association with severe asthma. Using a simplified approach, comorbid conditions can be grouped as either airway-related or airway-unrelated. In this context, we briefly outline prevalence, putative mechanisms, evidence for intervention, diagnosis and management (Box 1).
A practical approach to managing comorbid conditions in severe asthma
Grouping comorbid conditions into either airway-related or airway-unrelated factors may help to simplify diagnosis and management. However, application in everyday clinical practice can be problematic, and an iterative, algorithmic approach modelled on identification first of common comorbid conditions may help general practitioners to diagnose, refer appropriately and, where possible, manage comorbid conditions in severe asthma. If symptoms persist, less common comorbid conditions should be considered. Box 2 illustrates this proposed algorithm.
Airway-related comorbid conditions
Allergic rhinitis
Prevalence: The prevalence of allergic rhinitis (AR) in severe asthma may be as high as 55–68%.6,7 AR is also associated with early onset of severe asthma.8-10
Pathophysiology: The unified airway theory proposes that upper and lower airways function as a single unit and is supported by several observations. Patients with symptoms of AR often have reduced lung function on spirometry;11 individuals with allergen sensitisation have a higher prevalence of both asthma and AR;10 and the severity of AR often parallels that of asthma.12
Evidence for intervention: Patients with AR and severe asthma have poorer quality of life,12 greater airway dysfunction and symptoms, and a greater number of hospital admissions.9 They also have increased use of inhaled corticosteroids and bronchodilators.13
Evaluation and impact of management: Diagnosis of AR is symptom-based, and there are several validated questionnaires with high specificity and sensitivity such as the sinonasal questionnaire (SNQ).14
Treating comorbid AR in patients with asthma may reduce hospitalisation.15 A few small-scale randomised controlled trials (RCTs) have shown that nasally administered corticosteroids in AR also improve asthma control,16,17 although not in patients already using inhaled corticosteroids for asthma.18 Anti-leukotriene compounds have been shown to reduce hospitalisation15 and improve lung function in patients with asthma and AR, and also to reduce the corticosteroid dose.19 To date, there are limited data in severe asthma. Allergen-specific immunotherapy has been shown to relieve symptoms and reduce the use of medications, and also to reduce bronchial hyper-reactivity in mild but not severe asthma.20
Chronic rhinosinusitis
Prevalence: The prevalence of chronic rhinosinusitis (CRS) ranges from 45–50% in severe asthma.8,21 CRS is an independent predictor of asthma exacerbations in severe asthma.22,23 Nasal polyps are associated with a more severe asthma phenotype.24
Pathophysiology: There is evidence that severe asthma and CRS have similar pathological abnormalities. Eosinophil inflammation and local IgE production may occur in both,25 and innate lymphoid cells in sinus mucosal biopsies in CRS are associated with high tissue and blood eosinophilia.26 The inflammatory profile of sinus mucosa and bronchial biopsies also correlate substantially in both mild and severe asthma.27
Evidence for intervention: The presence of CRS is associated with lower asthma-related quality of life.28,29 CRS is also associated with poorer control and more exacerbations of asthma.28
Evaluation and impact of management: The European Position Paper on Rhinosinusitis and Nasal Polyps questionnaire is strongly associated with positive endoscopic findings in CRS.30 The SNQ also has high sensitivity and specificity for CRS.14
Nasal corticosteroids are considered first-line therapy in CRS treatment, with reduced asthma symptoms and improved control described in small RCTs.31 There is limited evidence for nasal lavage and low dose macrolides.31 Cohort studies have shown reduced asthma symptoms32 and improved control33 after sinus surgery. Anti-IgE therapy may yield improved outcomes,34 but there is a need for further studies.
Vocal cord dysfunction
Prevalence: The prevalence of VCD in asthma may range from 19% to 50% depending on severity. Our own data suggested that up to a third of patients with severe asthma have coexisting VCD.22,35,36
Pathophysiology: The causes of VCD are likely to be multifactorial. Dysfunctional breathing patterns may lead to laryngeal hyper-responsiveness followed by development of VCD. Several other conditions such as gastro-oesophageal reflux disease (GORD), anxiety and psychological disorders may contribute.37
Evidence for intervention: Due to symptom overlap, patients with VCD are often inaccurately diagnosed as having asthma, or as having severe asthma. This may lead to hospitalisation and inappropriate corticosteroid therapy.38
Evaluation and impact of management: Direct visualisation of paradoxical vocal cord movements (PVCM) is considered to be the gold standard for diagnosis. Our studies have used dynamic computed tomography of the larynx to diagnose VCD.36 Surrogate measures of laryngeal dysfunction include attenuation of the inspiratory flow loop on spirometry, but changes may be non-specific.39
Several questionnaires can be used, but sensitivity and specificity are questionable.40-42 The Vocal Cord Dysfunction Questionnaire (VCDQ) can be used to assess response to treatment.40 In asthma, the Pittsburgh VCD Index has a reported sensitivity of 0.83 and specificity of 0.95 for diagnosing laryngoscopy-proven VCD,41 but this has not been verified.
Treating VCD involves a multidisciplinary approach. Speech therapy is considered the mainstay of treatment43 and can relieve symptoms and reduce PVCM.40,44 Identifying and treating comorbid conditions (GORD, sinusitis, asthma) can yield improvement.43,45 As yet no convincing data exist to support use of continuous positive airway pressure (CPAP) therapy, botulinum toxin46 or psychotherapy.47 Benefits of treating VCD itself and the impact on asthma outcomes have not been determined.
Dysfunctional breathing
Prevalence: Dysfunctional breathing is a term describing breathing disorders where chronic changes in breathing pattern result in dyspnoea and other symptoms in the absence or in excess of the magnitude of physiological respiratory disease. As there is no established definition of dysfunctional breathing (DB), estimates of prevalence vary. In one study, DB was detected in up to 30% of patients with asthma; more so in those with difficult-to-treat asthma.22,48,49
Pathophysiology: Mechanisms are poorly understood but may be related to hypocapnia,50 thoraco-abdominal breathing asynchrony,51 or hyperventilation.52 Asthma has been linked to panic disorders49 which in turn are strongly associated with hyperventilation.53
Evidence for intervention: Patients with DB report a poorer asthma quality of life and asthma control, independent of airway hyper-responsiveness or airway inflammation.48
Evaluation and impact of management: The most widely used tool for assessing DB is the Nijmegen Questionnaire.54
Breathing techniques taught by a physiotherapist relieve respiratory symptoms without significant benefits on respiratory function.55,56 Breathing exercises have been shown to reduce symptoms57 and, in conjunction with thoracic muscle massage (Lotorp method), can relieve symptoms and improve peak expiratory flow (PEF), but not forced expiratory volume in 1 second (FEV1).58 A meta-analysis was unable to provide definitive conclusions about asthma outcomes with breathing exercises (due to variations in study methods), but there was a trend towards a positive effect.59 The benefits of psychological intervention on asthma outcomes have not been evaluated.
Allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitisation
Prevalence: The prevalence of allergic bronchopulmonary aspergillosis (ABPA) in severe asthma may be as high as 2.5%.60 In patients with asthma who have significantly elevated IgE (> 1000 IU/mL), the prevalence of ABPA increases to 15%.61 Isolation of fungus from the airway of patients with severe asthma and fungal sensitisation is common (up to 70%), suggestive of an association between fungal colonisation and both sensitisation and severity of asthma.62 This has led to the use of the term “severe asthma with fungal sensitisation” (SAFS) to describe sensitisation to Aspergillus in severe asthma in the absence of other features of ABPA.
Pathophysiology: ABPA is considered to be an immune response acting via type-2 T-lymphocyte pathways (Th2 pathways) in lung disease. Th2 CD4+ cell responses to Aspergillus antigens are seen in bronchoalveolar lavage and systemically.63 Sensitisation to Aspergillus increases the risk of adult-onset asthma.64
Evidence for intervention: ABPA is associated with persistent asthma, and there is a significant association between Aspergillus fumigatus IgE sensitisation, colonisation, and impaired post-bronchodilator FEV1.65
Evaluation and impact of management: Diagnostic criteria for SAFS have been proposed66 and include: predisposing condition such as asthma or cystic fibrosis; Aspergillus skin test positivity or detectable IgE levels against A. fumigatus; elevated total serum IgE concentration (> 1000 IU/mL); and one of: precipitating serum antibodies to A. fumigatus; radiographic pulmonary opacities; or a total eosinophil count of > 500 cells/µL.
Although improvements in asthma outcomes have not been demonstrated in high-quality studies, treatment of ABPA is recommended in severe asthma.67 The mainstay of ABPA treatment is oral corticosteroids.68 Adjunctive antifungal agents have been shown to reduce oral corticosteroid requirements, with itraconazole recommended after meta-analysis of 12 trials. (Suggested treatment regimens vary but most suggest an itraconazole course of 4–6 months duration69). Voriconazole and posaconazole are also effective, even as second-line agents in patients who do not respond to itraconazole,70 although there is a need for larger prospective studies. The impact of antifungal treatment in SAFS is uncertain, with mixed results in two RCTs.71 International guidelines suggest no treatment in the absence of other characteristics of ABPA.4 The anti-IgE monoclonal antibody treatment omalizumab may reduce exacerbations in ABPA.72
Bronchiectasis
Prevalence: The prevalence of bronchiectasis is significantly higher in severe asthma (range 24–40%)73,74 than in milder disease (3%).75
Pathophysiology: Impaired mucociliary clearance and increased bronchial secretions may lead to airway obstruction and airflow limitation. This in turn will predispose patients to exacerbations of their asthma.76
Evidence for intervention: Patients with asthma who have bronchiectasis have more exacerbations, hospitalisations,77 and more chronic respiratory failure.75
Evaluation and impact of management: High resolution computed tomography is the diagnostic modality of choice in bronchiectasis.78
Treatment for bronchiectasis includes airway clearance,79 exercise,80 and inhaled hyperosmolar agents.81,82 Regular macrolide use may reduce exacerbations in patients with bronchiectasis,83 without benefits on lung function.84 Despite the relatively high prevalence of bronchiectasis in severe asthma, to date, evidence to support specific treatments is lacking.
Chronic obstructive pulmonary disease
Prevalence: Chronic obstructive pulmonary disease (COPD) prevalence in severe asthma may be as high as 15–20%,85 but estimates are limited.
Pathophysiology: There is considerable overlap in the airway inflammation observed in severe asthma and COPD.86 Moreover, patients with asthma who smoke have increased numbers of neutrophils in the airways and corticosteroid resistance, as is characteristic of COPD.87,88
Evidence for intervention: Patients with asthma and COPD are more often hospitalised,89 have greater health impairment90 and more frequent exacerbations91,92 than for either condition alone.
Evaluation and impact of management: There is a limited body of evidence to inform management of patients with asthma and COPD. Smoking cessation is essential, and inhaled corticosteroids and bronchodilators remain the mainstay of treatment.93 Due to the important role of corticosteroids in uncontrolled asthma, the Global Initiative for Asthma (GINA) guidelines3 suggest treatment with inhaled corticosteroid (ICS) and adjunctive long-acting ß-agonist (LABA) therapy, with avoidance of LABA monotherapy. Several large high-quality RCTs have shown improvement in lung function and reduced risk of exacerbations when a long-acting muscarinic antagonist (LAMA), tiotropium, is added to ICS/LABA combination therapy, with other LAMAs requiring further study.94 A meta-analysis of eight studies (including four large RCTs) did not recommend LAMA over LABA add-on therapy to ICS.95 It should be noted that these studies generally included patients with asthma of mild to moderate severity and not those with typical asthma–COPD overlap.
Airway-unrelated comorbid conditions
Gastro-oesophageal reflux disease
Prevalence: GORD is common in patients with asthma, particularly severe asthma, with a reported prevalence of 46–63%.24,96,97 A study of 24-hour oesophageal pH testing in patients with asthma found that 51% of patients had abnormal results.97
Pathophysiology: Acid in the oesophagus may produce bronchoconstriction through increased vagal tone,98,99 and GORD increases bronchial hyper-responsiveness, with a dose–response relationship.100
The association between asthma and GORD may be bidirectional. Methacholine-induced bronchoconstriction has been found to increase the rate of transient lower oesophageal sphincter relaxation and reflux episodes.101 Additionally, the use of oral corticosteroids may exacerbate GORD.102
Evidence for intervention: GORD is associated with poorer asthma control and asthma-related quality of life22,103,104 and it is an independent predictor of asthma exacerbations.105,106
Evaluation and impact of management: Empiric therapy with a proton pump inhibitor (PPI) twice-daily is the recommended initial step in patients with symptoms of GORD.107 Improvement of asthma and GORD during a 3-month trial of PPI therapy is considered diagnostic of GORD-triggered asthma.108
RCTs of twice-daily PPI in patients with moderate-to-severe asthma and symptomatic GORD have shown a benefit on asthma quality of life and exacerbations,109 as well as minor improvements in PEF110 and FEV1111 However, treatment of asymptomatic gastro-oesophageal reflux has not been shown to improve asthma control112 or PEF.110 Referral to a gastroenterologist is indicated if GORD is not controlled on twice-daily PPI or if the patient has red flag symptoms (dysphagia, odynophagia, involuntary weight loss or anaemia).113
There is insufficient evidence to guide the use of anti-reflux surgery in patients with GORD-related asthma and there are no RCTs comparing the effectiveness of anti-reflux surgery to therapy with PPIs.114
Obesity
Prevalence: Obesity occurs frequently in patients with asthma. The rise in the prevalence of asthma has paralleled that of the obesity epidemic.115 The prevalence of obesity, defined as body mass index = 30, is 21–48% in patients with severe asthma.24,116,117
Epidemiological studies have shown that obesity is a predictor of asthma prevalence and incidence,118,119 and a meta-analysis found that the odds ratio of new-onset asthma is about 2.0 for obese compared with normal-weight subjects.118 Finally, obesity-associated late-onset asthma appears to represent a distinct clinical phenotype of severe asthma, with a preponderance in women.116,120,121
Pathophysiology: Mechanistic links between obesity and asthma are not understood. Impaired thoracic and airway mechanics related to breathing at lower lung volumes and with smaller tidal volumes in obese patients appears to affect airway function.122 Chronic systemic inflammation associated with obesity may be linked to airway inflammation.123 Interestingly, the association between obesity and asthma is stronger in non-allergic adults,124 suggesting that the association between obesity and severe asthma is not likely to be mediated by type-2 airway inflammation.
Evidence for intervention: Obesity is independently associated with asthma severity and poorer asthma outcomes.125,126 Obese patients are less responsive to asthma treatments.127,128 Obesity may also be an adverse consequence of systemic corticosteroid therapy. Obese patients with severe asthma are more likely to be on maintenance or frequent bursts of oral corticosteroid therapy.116,121
Evaluation and impact of management: Despite the association between obesity and asthma, there is a paucity of high-quality evidence to support the effectiveness of weight loss. A 2012 Cochrane review of trials of nonsurgical weight loss interventions concluded that the benefit on asthma control remains uncertain.115
More recently, however, a small randomised trial of dietary restriction and exercise found that a 5–10% weight loss resulted in small but clinically important improvements in asthma control and quality of life.129 Another recent randomised trial demonstrated that exercise and dietary modification led to greater weight loss at 3 months than dietary modification alone. This was associated with clinically significant improvements in asthma control, exacerbations and exercise capacity.130 A referral to a dietitian is essential, ideally within a multidisciplinary severe asthma service.131
Exercise training is well tolerated in asthma and improves aerobic exercise capacity and health-related quality of life.132 Exercise also appears to be directly beneficial to asthma control.129
Although bariatric surgery is the most effective method of achieving weight loss,133 there are no randomised trials assessing its impact on asthma outcomes.
Obstructive sleep apnoea
Prevalence: Cross-sectional studies of patients attending asthma clinics have reported that 39% have a high risk of obstructive sleep apnoea (OSA) based on the Berlin Questionnaire, a well validated screening tool.22,134 A meta-analysis of studies using polysomnography noted an OSA prevalence of 49.5% in the adult asthma population.135 Among patients with severe asthma, the prevalence is even higher, reported to be up to 88–96% in two small prospective studies.136,137
Pathophysiology: It is likely that OSA impacts on asthma through multiple mechanisms. Repetitive upper airway obstruction and hypoxia may induce airway inflammation, oxidative stress and systemic inflammation.138-141 There may also be vagally-mediated airway hyper-responsiveness secondary to upper airway collapse.142 Sleep apnoea may cause altered thoracic mechanics and intrathoracic pressures, which affect bronchial tone.140
The relationship between OSA and asthma could be bidirectional. Nocturnal asthma symptoms may exacerbate sleep apnoea and contribute to sleep disruption.140 Another potential mechanism is the effect of corticosteroids on the upper airway.137,143
Evidence for intervention: OSA is associated with worse asthma symptoms, even after controlling for obesity, GORD and nasal disease.144,145 Patients with severe or difficult-to-treat asthma and comorbid OSA have poorer asthma control and quality of life.22,146 Moreover, 47% of patients with asthma have excessive daytime sleepiness, and OSA may have an additional impact on symptom burden and impaired quality of life, beyond its effects on asthma control.147
Evaluation and impact of management: Patients with severe asthma should be routinely screened for OSA.3 The Berlin Questionnaire and STOP-Bang are validated tools.148,149 Screening is particularly important for asthma patients with comorbid obesity or nasal disease, since these patients are at higher risk for OSA.150 It is therefore recommended that patients at high risk for OSA be referred to a sleep physician for further assessment as appropriate.
An observational study of patients with asthma and moderate-to-severe OSA commenced on CPAP therapy showed small but clinically significant improvements in asthma control and quality of life at 6 months compared with baseline.151 This has not been studied in RCTs.
Anxiety and depression
Prevalence: Estimates of the prevalence of anxiety and depression in patients with asthma vary widely. In the general asthma population, the reported prevalence is 11–37% for anxiety and 11–18% for depression.152-154 Among patients with severe uncontrolled asthma, 81% had significant anxiety symptoms and 31% had symptoms of depression.155
Pathophysiology: Patients with severe asthma experience more psychological distress, worse cognitive dysfunction, and worse anxiety than those with moderate asthma. They also have more difficulty coping with their disease and have more problems with adherence to treament.156 Importantly, patients with severe prednisolone-dependent asthma are 3.5 times more likely to have depressive symptoms and 2.5 times more likely to have anxiety symptoms than patients with mild-to-moderate asthma.157
Evidence for intervention: Depression is associated with greater asthma severity, worse asthma control,112,158 poorer asthma-specific quality of life, and an increased risk of hospitalisation for asthma.153 Anxiety is associated with poor asthma control.152 Depression is also an independent predictor of poor adherence to treatment with asthma medications.159 A study of patients with difficult-to-treat asthma found that psychological dysfunction is associated with an almost 11-fold increase in the risk of frequent exacerbations. It was the strongest predictor out of 13 potential risk factors studied.23 In addition, depression is associated with an increased risk of long-term or permanent work disability in patients with asthma.160
Evaluation and impact of management: Screening for anxiety and depression is recommended in all patients with severe asthma.3 The Hospital Anxiety and Depression Scale (HADS) is a self-assessment questionnaire which is well validated and easily administered.161 Patients with significant symptoms of anxiety and/or depression should be referred to a psychologist, ideally within a multidisciplinary severe asthma service.131
There is a paucity of data on the effectiveness of treatment for anxiety and depression on asthma outcomes. A systematic review of psycho-educational interventions in patients with difficult-to-treat asthma found weak evidence that it may reduce hospital admissions. However, the quality of the studies was poor and there was variability in the interventions used.162 A randomised trial of cognitive behavioural therapy for asthma-specific anxiety found that this was effective in reducing anxiety, but other asthma-related outcomes were not studied.163
Conclusion
Comorbid conditions are frequently a treatable component of severe asthma. This requires careful clinical assessment, special investigations where indicated and, sometimes, referral to specialists or specialised clinics. General practitioners have a crucial role to play in recognising the range of comorbid conditions, directing investigations and referrals and, ultimately, implementing optimised management. Coordinated, holistic care of patients with severe asthma and a spectrum of comorbidities is often not feasible within specialist respiratory practice; effective collaboration with general practitioners, who may be better placed to oversee optimal management across all domains, is essential.164
Box 1 – Prevalence, associated asthma phenotypes and management options for common comorbid conditions in severe asthma
|
Comorbidity |
Prevalence in severe asthma |
Most commonly associated asthma phenotype |
Management options |
||||||||||||
|
|
|||||||||||||||
|
Airway-related comorbid conditions |
|
|
|||||||||||||
|
Allergic rhinitis |
55–68% |
Early onset allergic asthma |
|
||||||||||||
|
Chronic rhinosinusitis with or without nasal polyposis |
45–50% |
Late onset eosinophilic asthma (particularly associated with nasal polyposis) |
|
||||||||||||
|
Vocal cord dysfunction |
19–50% |
Not associated with an asthma phenotype |
|
||||||||||||
|
Dysfunctional breathing |
24–30% |
Late onset non-eosinophilic asthma |
|
||||||||||||
|
Allergic bronchopulmonary aspergillosis or severe asthma with fungal sensitisation |
2.5% |
Late onset allergic asthma |
|
||||||||||||
|
Bronchiectasis |
24–40% |
Not associated with an asthma phenotype |
|
||||||||||||
|
Smoking and chronic obstructive pulmonary disease |
Variable, up to 15–20% |
Not associated with an asthma phenotype |
|
||||||||||||
|
Airway-unrelated comorbid conditions |
|
|
|||||||||||||
|
Gastro-oesophageal reflux disease |
46–63% |
Not associated with an asthma phenotype |
|
||||||||||||
|
Obesity |
21–48% |
Late onset non-eosinophilic asthma |
|
||||||||||||
|
Obstructive sleep apnoea |
Up to 88–96% on polysomnography |
Not associated with an asthma phenotype |
|
||||||||||||
|
Anxiety and depression |
Anxiety 81% |
Not associated with an asthma phenotype |
|
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Proposed iterative approach to assessing comorbid conditions in patients with severe asthma
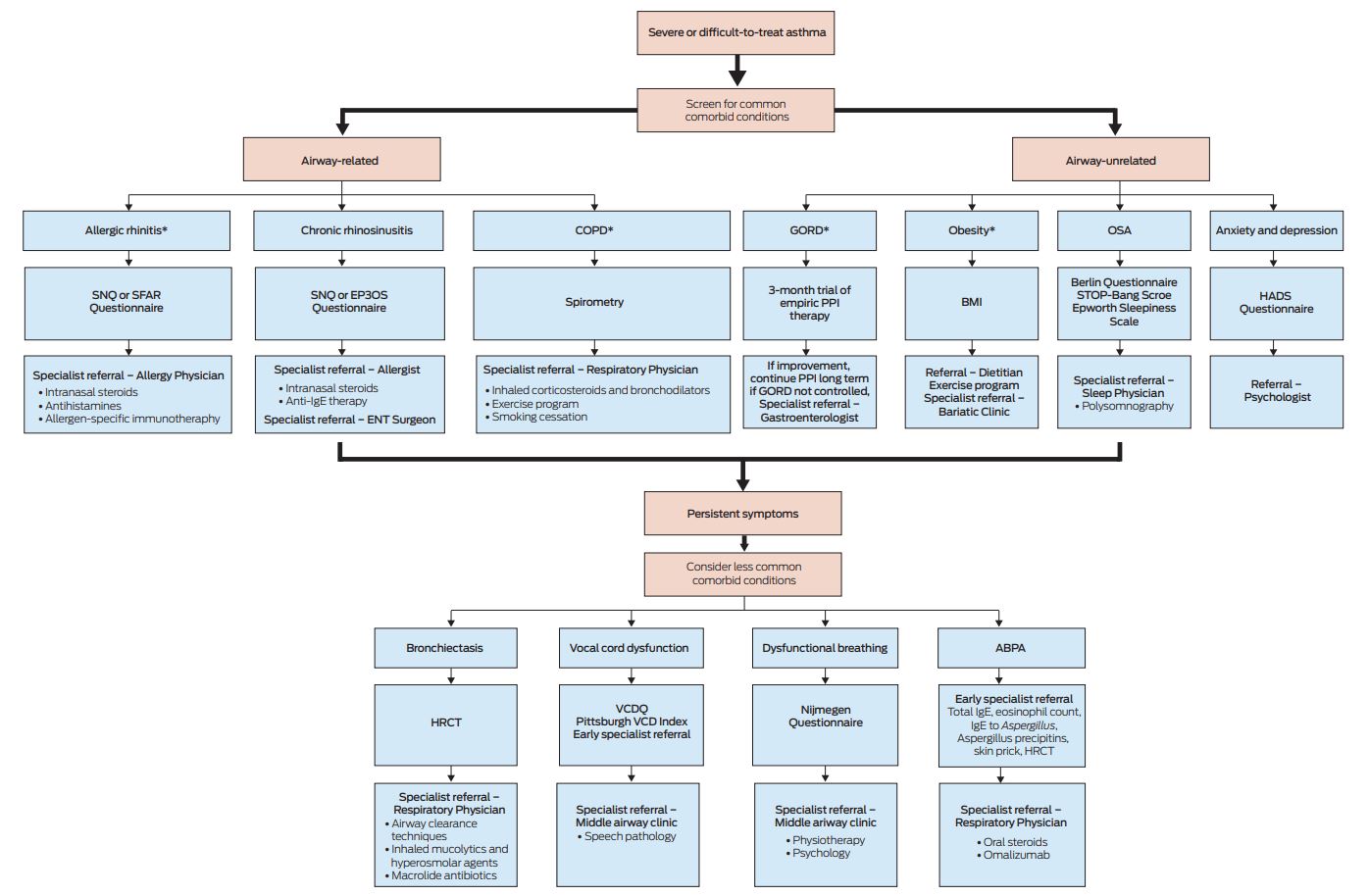
*Magement may be appropriately initiated in primary care. Specialist referral is suggested at the discretion of the primary care physician or if treatment responses are unsatisfactory. Early specialist referral for diagnosis and management is generally indicated for less common co-morbid conditions.
ABPA = allergic bronchopulmonary aspergillosis; BMI = body mass index; COPD = chronic obstructive pulmonary disease; ENT = ear nose and throat; EP3OS = European Position Paper on Rhinosinusitis and Nasal Polyps questionnaire; GORD = gastro-oesophageal reflux disease; HADS = Hospital Anxiety and Depression Scale; HRCT = high-resolution computed tomography; OSA = obstructive sleep apnoea; PPI = proton pump inhibitor; SFAR = score for allergic rhinitis; SNQ = Sino-Nasal Questionnaire; VCD = vocal cord dysfunction; VCDQ = vocal cord dysfunction questionnaire.
Provenance: Commissioned; externally peer reviewed.
- Philip G Bardin
- Jhanavi Rangaswamy
- Shaun W Yo
- Monash Lung and Sleep, Monash Hospital and University, Melbourne, VIC
No relevant disclosures.
- 1. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 2017; 377: 965-976.
- 2. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410-419.
- 3. Global Initiative for Asthma. Global Strategy for asthma management and prevention 2018. https://ginasthma.org/ (viewed Feb 2018)
- 4. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343-373.
- 5. Tay TR, Lee J W-Y, Hew M. Diagnosis of severe asthma. Med J Aust 2018; 209 (2 Suppl): S3-S10.
- 6. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119: 405-413.
- 7. Ohta K, Bousquet PJ, Aizawa H, et al. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy 2011; 66: 1287-1295.
- 8. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181: 315-323.
- 9. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218-224.
- 10. Warm K, Hedman L, Lindberg A, et al. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol 2015; 136: 1559-1565.
- 11. Ciprandi G, Cirillo I, Pistorio A. Impact of allergic rhinitis on asthma: effects on spirometric parameters. Allergy 2008; 63: 255-260.
- 12. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy 2010; 65: 1290-1297.
- 13. Wu TJ, Chen BY, Lee YL, et al. Different severity and severity predictors in early-onset and late-onset asthma: a Taiwanese population-based study. Respiration 2015; 90: 384-392.
- 14. Dixon AE, Sugar EA, Zinreich SJ, et al. Criteria to screen for chronic sinonasal disease. Chest 2009; 136: 1324-1332.
- 15. Dal Negro R, Piskorz P, Vives R, et al. Healthcare utilisation and costs associated with adding montelukast to current therapy in patients with mild to moderate asthma and co-morbid allergic rhinitis: PRAACTICAL study. Pharmacoeconomics 2007; 25: 665-676.
- 16. Scichilone N, Arrigo R, Paterno A, et al. The effect of intranasal corticosteroids on asthma control and quality of life in allergic rhinitis with mild asthma. J Asthma 2011; 48: 41-47.
- 17. Baiardini I, Villa E, Rogkakou A, et al. Effects of mometasone furoate on the quality of life: a randomized placebo-controlled trial in persistent allergic rhinitis and intermittent asthma using the Rhinasthma questionnaire. Clin Exp Allergy 2011; 41: 417-423.
- 18. Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy 2013; 68: 569-579.
- 19. Price DB, Swern A, Tozzi CA, et al. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy 2006; 61: 737-742.
- 20. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev 2010; (8): CD001186.
- 21. ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol 2002; 109: 621-626.
- 22. Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016; 21: 1384-1390.
- 23. ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26: 812-818.
- 24. Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46: 1308-1321.
- 25. Dixon AE. Rhinosinusitis and asthma: the missing link. Curr Opin Pulm Med 2009; 15: 19-24.
- 26. Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy 2015; 45: 394-403.
- 27. Håkansson K, Bachert C, Konge L, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: the United Airways concept further supported. PloS One 2015; 10: e0127228.
- 28. Dixon AE, Kaminsky DA, Holbrook JT, et al. Allergic rhinitis and sinusitis in asthma. Chest 2006; 130: 429-435.
- 29. Ek A, Middelveld RJM, Bertilsson H, et al. Chronic rhinosinusitis in asthma is a negative predictor of quality of life: results from the Swedish GA2LEN survey. Allergy 2013; 68: 1314-1321.
- 30. Tomassen P, Newson RB, Hoffmans R, et al. Reliability of EP3OS symptom criteria and nasal endoscopy in the assessment of chronic rhinosinusitis–a GA(2) LEN study. Allergy 2011 66: 556-561.
- 31. Ragab S, Scadding GK, Lund VJ, et al. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J 2006; 28: 68-74.
- 32. Batra PS, Kern RC, Tripathi A, et al. Outcome analysis of endoscopic sinus surgery in patients with nasal polyps and asthma. Laryngoscope 2003; 113: 1703-1706.
- 33. Chen FH, Zuo KJ, Guo YB, et al. Long-term results of endoscopic sinus surgery-oriented treatment for chronic rhinosinusitis with asthma. Laryngoscope 2014; 124: 24-28.
- 34. Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013; 131: 110-116.
- 35. Yelken K, Yilmaz A, Guven M, et al. Paradoxical vocal fold motion dysfunction in asthma patients. Respirology 2009; 14: 729-733.
- 36. Low K, Lau KK, Holmes P, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med 2011; 184: 50-56.
- 37. Bardin PG, Low K, Ruane L, et al. Controversies and conundrums in vocal cord dysfunction. Lancet Respir Med 2017; 5: 546-548.
- 38. Newman KB, Mason UG 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med 1995; 152: 1382-1386.
- 39. Watson MA, King CS, Holley AB, et al. Clinical and lung-function variables associated with vocal cord dysfunction. Respir Care 2009; 54: 467-473.
- 40. Fowler SJ, Thurston A, Chesworth B, et al. The VCDQ–a Questionnaire for symptom monitoring in vocal cord dysfunction. Clin Exp Allergy 2015; 45: 1406-1411.
- 41. Traister RS, Fajt ML, Landsittel D, et al. A novel scoring system to distinguish vocal cord dysfunction from asthma. J Allergy Clin Immunol Pract 2014; 2: 65-69.
- 42. Pinto LHE, Aun MV, Cukier-Blaj S, et al. Vocal cord dysfunction diagnosis may be improved by a screening check list. Allergol Int 2016; 65: 180-185.
- 43. Kenn K, Balkissoon R. Vocal cord dysfunction: what do we know? Eur Respir J 2011; 37: 194-200.
- 44. Murry T, Tabaee A, Aviv JE. Respiratory retraining of refractory cough and laryngopharyngeal reflux in patients with paradoxical vocal fold movement disorder. Laryngoscope 2004; 114: 1341-1345.
- 45. Bucca CB, Bugiani M, Culla B, et al. Chronic cough and irritable larynx. J Allergy Clin Immunol 2011; 127: 412-419.
- 46. Maillard I, Schweizer V, Broccard A, et al. Use of botulinum toxin type A to avoid tracheal intubation or tracheostomy in severe paradoxical vocal cord movement. Chest 2000; 118: 874-877.
- 47. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope 2008; 118: 740-747.
- 48. Veidal S, Jeppegaard M, Sverrild A, et al. The impact of dysfunctional breathing on the assessment of asthma control. Respir Med 2017; 123: 42-47.
- 49. Thomas M, McKinley RK, Freeman E, et al. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ 2001; 322: 1098-1100.
- 50. Osborne CA, O’Connor BJ, Lewis A, et al. Hyperventilation and asymptomatic chronic asthma. Thorax 2000; 55: 1016-1022.
- 51. Courtney R, van Dixhoorn J, Greenwood KM, et al. Medically unexplained dyspnea: partly moderated by dysfunctional (thoracic dominant) breathing pattern. J Asthma 2011; 48: 259-265.
- 52. Boulding R, Stacey R, Niven R, et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev 2016; 25: 287-294.
- 53. Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. Int J Psychophysiol 2010; 78: 68-79.
- 54. van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985; 29: 199-206.
- 55. Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007; 62: 1039-1042.
- 56. Thomas M, McKinley RK, Mellor S, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax 2009; 64: 55-61.
- 57. Courtney R, Cohen M. Investigating the claims of Konstantin Buteyko, MD, Ph.D.: the relationship of breath holding time to end tidal CO2 and other proposed measures of dysfunctional breathing. J Altern Complement Med 2008; 14: 115-123.
- 58. Lowhagen O, Bergqvist P. Physiotherapy in asthma using the new Lotorp method. Complement Ther Clin Pract 2014; 20: 276-279.
- 59. Freitas DA, Holloway EA, Bruno SS, et al. Breathing exercises for adults with asthma. Cochrane Database Syst Rev 2013; (10): CD001277.
- 60. Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013; 51: 361-370.
- 61. Tay TR, Bosco J, Gillman A, et al. Coexisting atopic conditions influence the likelihood of allergic bronchopulmonary aspergillosis in asthma. Ann Allergy Asthma Immunol 2016; 117: 29-32.
- 62. Farrant J, Brice H, Fowler S, et al. Fungal sensitisation in severe asthma is associated with the identification of Aspergillus fumigatus in sputum. J Asthma 2016; 53: 732-735.
- 63. Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37 Suppl 3: S225-S264.
- 64. Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol 2006; 117: 642-648.
- 65. Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med 2010; 182: 1362-1368.
- 66. Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43: 850-873.
- 67. Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 2017; 22: 651-661.
- 68. Greenberger PA, Bush RK, Demain JG, et al. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014; 2: 703-708.
- 69. Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev 2004; (3): CD001108.
- 70. Chishimba L, Niven RM, Cooley J, et al. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma 2012; 49: 423-433.
- 71. Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 2009; 179: 11-18.
- 72. Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015; 3: 192-199.
- 73. Bisaccioni C, Aun MV, Cajuela E, et al. Comorbidities in severe asthma: frequency of rhinitis, nasal polyposis, gastroesophageal reflux disease, vocal cord dysfunction and bronchiectasis. Clinics (Sao Paulo) 2009; 64: 769-773.
- 74. Gupta S, Siddiqui S, Haldar P, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest 2009; 136: 1521-1528.
- 75. Oguzulgen IK, Kervan F, Ozis T, et al. The impact of bronchiectasis in clinical presentation of asthma. South Med J 2007; 100: 468-471.
- 76. Truong T. The overlap of bronchiectasis and immunodeficiency with asthma. Immunol Allergy Clin North Am 2013; 33: 61-78.
- 77. Kang HR, Choi GS, Park SJ, et al. the effects of bronchiectasis on asthma exacerbation. Tuberc Respir Dis (Seoul) 2014; 77: 209-214.
- 78. Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65 Suppl 1: i1-i58.
- 79. Lee AL, Burge A, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2013: CD008351.
- 80. Lee AL, Hill CJ, Cecins N, et al. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis–a randomised controlled trial. Respir Res. 2014; 15: 44.
- 81. Nicolson CH, Stirling RG, Borg BM, et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 661-667.
- 82. Hill AT, Welham S, Reid K, et al. British Thoracic Society national bronchiectasis audit 2010 and 2011. Thorax 2012; 67: 928-930.
- 83. Wu Q, Shen W, Cheng H, et al. Long-term macrolides for non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. Respirology 2014; 19: 321-329.
- 84. Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380: 660-667.
- 85. Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014; 6: S146-S151.
- 86. Cowan DC, Cowan JO, Palmay R, et al. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010; 65: 384-390.
- 87. Chalmers GW, Macleod KJ, Little SA, et al. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax 2002; 57: 226-230.
- 88. Chaudhuri R, Livingston E, McMahon AD, et al. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003; 168: 1308-1311.
- 89. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest 2008; 134: 14-19.
- 90. Pleasants RA, Ohar JA, Croft JB, et al. Chronic obstructive pulmonary disease and asthma-patient characteristics and health impairment. COPD 2014; 11: 256-266.
- 91. Menezes AMB, Montes de Oca M, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014; 145: 297-304.
- 92. Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011; 12: 127-127.
- 93. Nakawah MO, Hawkins C, Barbandi F. Asthma, chronic obstructive pulmonary disease (COPD), and the overlap syndrome. J Am Board Fam Med 2013; 26: 470-477.
- 94. Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 2017; 358: j3772.
- 95. Kew KM, Evans DJ, Allison DE, et al. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst Rev 2015; (6): Cd011438.
- 96. Chipps BE, Haselkorn T, Paknis B, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol 2018; 141:1590-1597.
- 97. Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut 2007; 56: 1654-1664.
- 98. Amarasiri DL, Pathmeswaran A, de Silva HJ, et al. Response of the airways and autonomic nervous system to acid perfusion of the esophagus in patients with asthma: a laboratory study. BMC Pulm Med 2013; 13: 33.
- 99. Schan CA, Harding SM, Haile JM, et al. Gastroesophageal reflux-induced bronchoconstriction. An intraesophageal acid infusion study using state-of-the-art technology. Chest 1994; 106: 731-737.
- 100. Vincent D, Cohen-Jonathan AM, Leport J, et al. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997; 10: 2255-2259.
- 101. Zerbib F, Guisset O, Lamouliatte H, et al. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med 2002; 166: 1206-1211.
- 102. Lazenby JP, Guzzo MR, Harding SM, et al. Oral corticosteroids increase esophageal acid contact times in patients with stable asthma. Chest 2002; 121: 625-634.
- 103. Cheung TK, Lam B, Lam KF, et al. Gastroesophageal reflux disease is associated with poor asthma control, quality of life, and psychological status in Chinese asthma patients. Chest 2009; 135: 1181-1185.
- 104. DiMango E, Holbrook JT, Simpson E, et al. Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med 2009; 180: 809-816.
- 105. Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: a Respiratory Effectiveness Group initiative. J Allergy Clin Immunol Pract 2017; 5: 1015-1024.e1018.
- 106. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med 2017; 195: 302-313.
- 107. Naik RD, Vaezi MF. Extra-esophageal gastroesophageal reflux disease and asthma: understanding this interplay. Expert Rev Gastroenterol Hepatol 2015; 9: 969-982.
- 108. Harding SM, Richter JE, Guzzo MR, et al. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 1996; 100: 395-405.
- 109. Littner MR, Leung FW, Ballard ED 2nd, et al. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128: 1128-1135.
- 110. Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 mg twice daily on asthma. Am J Respir Crit Care Med 2006; 173: 1091-1097.
- 111. Kiljander TO, Junghard O, Beckman O, et al. Effect of esomeprazole 40 mg once or twice daily on asthma. Am J Respir Crit Care Med 2010; 181: 1042-1048.
- 112. Mastronarde JG, Anthonisen NR, Castro M, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360: 1487-1499.
- 113. DeVault KR, Castell DO, American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100: 190-200.
- 114. Sidwa F, Moore AL, Alligood E, et al. Surgical Treatment of extraesophageal manifestations of gastroesophageal reflux disease. World J Surg 2017; 41: 2566-2571.
- 115. Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev 2012; (7): CD009339.
- 116. Gibeon D, Batuwita K, Osmond M, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest 2013; 143: 406-414.
- 117. van Veen IH, Ten Brinke A, Sterk PJ, et al. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy 2008; 63: 570-574.
- 118. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175: 661-666.
- 119. Young SY, Gunzenhauser JD, Malone KE, et al. Body mass index and asthma in the military population of the northwestern United States. Arch Intern Med 2001; 161: 1605-1611.
- 120. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218-224.
- 121. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181: 315-323.
- 122. Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med 2013; 107: 1287-1300.
- 123. Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 2005; 115: 925-927.
- 124. Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest 2006; 130: 890-895.
- 125. Mosen DM, Schatz M, Magid DJ, et al. The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol 2008; 122: 507-511.e506.
- 126. Taylor B, Mannino D, Brown C, et al. Body mass index and asthma severity in the National Asthma Survey. Thorax 2008; 63: 14-20.
- 127. Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 2007; 101: 2240-2247.
- 128. Dixon AE, Shade DM, Cohen RI, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma 2006; 43: 553-558.
- 129. Scott HA, Gibson PG, Garg ML, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013; 43: 36-49.
- 130. Freitas PD, Ferreira PG, Silva AG, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 32-42.
- 131. McDonald VM, Vertigan AE, Gibson PG. How to set up a severe asthma service. Respirology 2011; 16: 900-911.
- 132. Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev 2013; (9): CD001116.
- 133. Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev 2009; (2): CD003641.
- 134. Auckley D, Moallem M, Shaman Z, et al. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med 2008; 9: 494-499.
- 135. Kong DL, Qin Z, Shen H, et al. Association of obstructive sleep apnea with asthma: a meta-analysis. Sci Rep 2017; 7: 4088.
- 136. Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol 2009; 124: 371-376.
- 137. Yigla M, Tov N, Solomonov A, et al. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003; 40: 865-871.
- 138. Carpagnano GE, Kharitonov SA, Resta O, et al. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 2002; 122: 1162-1167.
- 139. Devouassoux G, Levy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 2007; 119: 597-603.
- 140. Mehra R, Redline S. Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol 2008; 121: 1096-1102.
- 141. Salerno FG, Carpagnano E, Guido P, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 2004; 98: 25-28.
- 142. Guilleminault C, Quera-Salva MA, Powell N, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J 1988; 1: 902-907.
- 143. Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med 2014; 10: 183-193.
- 144. Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010; 138: 543-550.
- 145. Teodorescu M, Polomis DA, Teodorescu MC, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma 2012; 49: 620-628.
- 146. Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract 2015; 3: 566-575.
- 147. Teodorescu M, Consens FB, Bria WF, et al. Correlates of daytime sleepiness in patients with asthma. Sleep Med 2006; 7: 607-613.
- 148. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131: 485-491.
- 149. Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: a tool to screen obstructive sleep apnea. Anesthesiology 2008; 108: 812-821.
- 150. Jiang RS, Liang KL, Hsin CH, et al. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology 2016; 54: 75-79.
- 151. Serrano-Pariente J, Plaza V, Soriano JB, et al. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 2017; 72: 802-812.
- 152. Ciprandi G, Schiavetti I, Rindone E, et al. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol 2015; 115: 408-414.
- 153. Eisner MD, Katz PP, Lactao G, et al. Impact of depressive symptoms on adult asthma outcomes. Ann Allergy Asthma Immunol 2005; 94: 566-574.
- 154. Kullowatz A, Kanniess F, Dahme B, et al. Association of depression and anxiety with health care use and quality of life in asthma patients. Respir Med 2007; 101: 638-644.
- 155. de Carvalho-Pinto RM, Cukier A, Angelini L, et al. Clinical characteristics and possible phenotypes of an adult severe asthma population. Respir Med 2012; 106: 47-56.
- 156. Lavoie KL, Bouthillier D, Bacon SL, et al. Psychologic distress and maladaptive coping styles in patients with severe vs moderate asthma. Chest 2010; 137: 1324-1331.
- 157. Amelink M, Hashimoto S, Spinhoven P, et al. Anxiety, depression and personality traits in severe, prednisone-dependent asthma. Respir Med 2014; 108: 438-444.
- 158. Goldney RD, Ruffin R, Fisher LJ, et al. Asthma symptoms associated with depression and lower quality of life: a population survey. Med J Aust 2003; 178: 437-441. <MJA full text>
- 159. Smith A, Krishnan JA, Bilderback A, et al. Depressive symptoms and adherence to asthma therapy after hospital discharge. Chest 2006; 130: 1034-1038.
- 160. Hakola R, Kauppi P, Leino T, et al. Persistent asthma, comorbid conditions and the risk of work disability: a prospective cohort study. Allergy 2011; 66: 1598-1603.
- 161. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361-370.
- 162. Smith JR, Mugford M, Holland R, et al. A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma. Health Technol Assess 2005; 9: iii-iv, 1-167.
- 163. Parry GD, Cooper CL, Moore JM, et al. Cognitive behavioural intervention for adults with anxiety complications of asthma: prospective randomised trial. Respir Med 2012; 106: 802-810.
- 164. Chung LP, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust 2018; 209 (2 Suppl): S34-S40.





Summary