Severe asthma is a complex and heterogeneous disease of the airways that is associated with an ongoing and burdensome symptom profile, frequent attacks of asthma and multiple comorbidities. It affects between 3% and 10% of individuals with an asthma diagnosis, and has a major impact on the lives of patients and their families.1,2 Unlike those with mild to moderate asthma, people with severe disease are usually refractory to standard treatment, have poor symptom control affecting their social and working life, and are at increased risk of death. In addition, severe asthma places a large financial burden on the health care system, society and individuals.1,2
As a result, severe asthma affects the health-related quality of life (HRQoL) of patients. HRQoL is defined as a multidimensional concept that includes domains related to physical, mental, emotional and social functioning. It focuses on the impact that health status has on the quality of life of individuals, going beyond direct measures of morbidity and mortality.3
The HRQoL burden for patients with severe asthma is frequently underappreciated. Current public health messages erroneously promote that individuals with asthma can live normal lives unaffected by asthma symptoms and attacks.4 While this might be true for those with mild to moderate asthma, it causes additional difficulty for individuals with severe disease, for whom poor symptom control, exacerbations, and impacts on social life, physical activity, work and school are often unavoidable. Improving the understanding of this burden among health care practitioners, policy makers and the community is important if we are to address the gaps in severe asthma management and outcomes.
In this article, we will highlight the impact that severe asthma has on HRQoL, discuss interventions that may improve it, and examine ways of assessing HRQoL in the clinic.
Understanding the patient’s perspective
HRQoL in severe asthma is impaired across all domains.2;5-7 Although the extent and nature of impairment varies between patients,8,9 compromised HRQoL may be one of the hallmarks of severe disease, with quality of life scores accurately distinguishing severe from non-severe asthma in children6 and adults.10 HRQoL may also deteriorate over time, particularly for those who experience serious attacks.11 Stories describing the impact of severe asthma on the lives of individuals are available online (http://toolkit.severeasthma.org.au/living-severe-asthma/overview and https://www.asthma.org.uk/advice/severe-asthma/your-stories-severe-asthma).
Most individuals with mild to moderate asthma are able to achieve adequate symptom control and avoid attacks of asthma through treatment with, and adherence to, standard guideline-based pharmacological therapy. However, people with severe asthma infrequently achieve this despite an often complex regimen, which, for example, involves the need to self-manage their disease using regular inhaled therapies with multiple devices, in addition to managing their therapy for multiple comorbidities.1 Due to the refractory nature of the disease, additional treatments such as oral corticosteroids, which often have significant side effects, are added to the already difficult regimen, and this can be an additional burden. Even this may not result in an obvious improvement in symptoms, and patients may remain at continued risk of severe attacks.
Our understanding of the impact of severe asthma on individuals’ lives — an area that was previously poorly explored — has recently expanded through qualitative research. An Australian study involving qualitative interviews with 25 people with severe asthma describes the impact of the disease and why it contributes such a major burden.4 In this study, four themes emerged from the data, including the “body as a hindrance”, the “burden of treatment”, being “alone with asthma” and “striving to adapt”. In describing the impact of her disease, a 54-year-old woman states: “I have lost in every facet of my life … My earning capacity, my self-esteem, my sense of achievement, my relationships. You name it, it’s been there”.4 This highlights the all-encompassing nature of severe asthma.
Biases about severe asthma and its perceived impact, or lack thereof, from health care professionals, the media, colleagues and the social networks of people with the condition also affect the HRQoL of individuals, and these perceptions need to be appropriately addressed.4,12 The Australian study also highlighted how public perception of asthma leads to people feeling alone, through confusing messages and general public misunderstanding. Participants refer to the isolating nature of television advertisements which portray people with asthma as being able to achieve anything:
“You have all these ads on telly with wonderful Australian cricketers that play world class cricket, and they take their puffer. ‘We have asthma but we can do anything’ … [People think], ‘So why can’t you do that?’” (60-year-old woman).4
Another powerful example of the misunderstanding people with severe asthma face about their condition was articulated by a patient with severe disease:
“I think it needs a bit more publicity. Because when you say to people ‘I’ve got severe asthma’ I think 90% of the population goes ‘Oh yeah, asthma. Every second person has that’. I don’t think there’s a comprehension there … [There] needs to be that acknowledgement that people with severe asthma are [not mild] asthmatics,” (38-year-old woman).4
These examples highlight the need for new public health messages that acknowledge the impact of severe asthma on patients’ quality of life and their ability to function.
A systematic review of five qualitative studies describing the experience of living with severe asthma confirms these findings, and provides further insight as to why people with severe asthma have such quality of life burden, with four interrelated subthemes described.13 In the theme “striving for autonomy through dealing with symptoms and treatment,” the burden of coping with symptoms is evident; while these relate to the continuous disease-specific symptoms of wheeze, breathlessness and cough, participants also highlight the panic and fear that they experience in relation to the unpredictability of their next attack.13
Treatment adherence is an important and probably misunderstood behaviour in severe asthma.14 Despite the frequency of symptoms and the known risk of severe attacks, suboptimal adherence is common in this population, with reported prevalence of suboptimal adherence of 44% in one cohort with severe asthma.15 Qualitative studies give some insight into this behaviour in severe asthma and the impact of treatment on quality of life. The burden of treatment and the adverse effects of medication were noted as significant issues by participants in all of the studies.4,13 Patients will frequently weigh up the costs and benefits of treatment in an attempt to lead a normal life. They discuss the negative and unwanted side effects of oral corticosteroids particularly, and the impact that a regular and complex regimen has on their lives:
“You’re a slave to this regimented ordeal you’ve got to go through every day” (54-year-old man).4
Comorbidities are more common in people with severe asthma than in populations with milder asthma.16 This is also important for the patient’s quality of life as prescribed treatment may be inappropriate or ineffective if asthma symptoms are aggravated or mimicked by a comorbid illness.17 Bardin and colleagues provide a comprehensive review on comorbidities in severe asthma in this issue.18
Quantifying the impact of severe asthma on health-related quality of life
Many of the factors that contribute to impaired HRQoL in severe asthma are shown in Box 1. Severe asthma is associated with frequent and intrusive symptoms (89% report daily wheeze, 56% cough, and 39% phlegm and shortness of breath).9 Asthma symptoms may lead to other more general symptoms, such as fatigue, exhaustion and poor sleep quality (reported in up to 94% of patients),9 as well as deficits in other quality of life domains, including discomfort, distress and functional limitation.4 Poor asthma control, frequent attacks, suboptimal medication adherence, and reduced lung function are all correlated with poor HRQoL.5,7,27
Major functional limitation is another feature reported by 70–100% of patients with severe asthma,8,9 and is characterised by decreased ability to complete daily activities (85%),16 physical activity limitation (69%)8, loss in productivity at work (73%)16 or study (64%),2 and limits in leisure and lifestyle (78%).9,28 From a quality of life perspective, one of the most prominent limitations is in physical activity, which spans from an inability to participate in sports and vigorous activities through to an inability to walk far from a bed or chair.9,29 Physical activity limitation is 2.6 times more common in uncontrolled, compared with controlled, moderate to severe asthma,29 and people with this disease walk over 2000 fewer steps per day than healthy controls, reaching a median of only 5362 (interquartile range, 3999–7817) steps per day.30 These limitations lead to people with severe asthma feeling isolated or “alone with asthma”.4,13 Most patients report that their disease prohibits activities they would like to do (78%),9 with many reporting limitations in pet ownership (49%), going out with friends (38%), taking holidays (28%) and limited job prospects (21%) or participation at school (14%).8
In terms of workplace productivity, people with severe asthma are more likely than those with milder asthma to be absent from work (27% v 18%) or feel impaired at work (73% v 43%).16 Limitation in employment has negative financial and emotional consequences, which, in addition to the high cost of asthma management, burden people with severe asthma and their families.4,31
Many more children and adults with severe asthma experience poor mental health compared with those with milder asthma (depression, 25% v 9%; anxiety, 38% v 30%),32 and report difficulties in relationships, stigma, and low satisfaction with life.2,4,9 Emotions and thoughts commonly described include worry, fear and panic (particularly regarding inability to breathe or not having medications when needed), sadness, worthlessness, frustration and irritability.4,9,33 These emotions and thoughts often generate negative coping strategies, such as avoidance of physical activity.4 Nevertheless, some patients with severe asthma do report adjusting to their life using positive coping strategies.4
Improving quality of life in severe asthma
There are several interventions aimed at improving quality of life in severe asthma, both directly and indirectly. These interventions include behavioural and pharmacological treatments, and may involve single targeted treatments or bundled interventions, which include the assessment and treatment of related comorbidities and risk factors.
Multidimensional assessment
To account for the heterogeneity of severe asthma, current guidelines34 recommend that individuals with severe disease undergo systematic or multidimensional assessment. A multidimensional assessment involves a coordinated series of investigations and evaluations designed to confirm the diagnosis, identify comorbidities relevant to severe asthma and determine risk factors.35 This includes airway assessments for bronchodilator reversibility, airflow limitation and airway inflammation;35 assessment of relevant comorbidities, such as anxiety, depression, gastro-oesophageal reflux disease and sinusitis; and assessment of important risk factors, such as smoking, obesity, self-management skills, inhaler device technique, treatment adherence and inhaler device polypharmacy.35 Results of the assessments identify clinical problems, which can then inform management decisions such as treatment and specialist referrals. While to date there are no published randomised controlled trials of this approach in severe asthma, a meta-analysis of three observational studies has reported improvements in HRQoL as well as improved asthma control and reduction in exacerbations for this system of care, up to a year after implementation36 (Box 2). Due to the complex nature of multidimensional assessment, it is unclear which aspects drive the improvements in HRQoL.
Non-pharmacological interventions and health-related quality of life
Non-pharmacological interventions are promising avenues for improving HRQoL in severe asthma (Box 1); however, much of the evidence to support these interventions has been developed in general asthma populations and not severe asthma specifically. Interventions such as education and self-management programs,37-39 psychological therapies40 and weight loss and lifestyle modification41-43 are associated with small to moderate improvements in HRQoL. Such interventions are also likely to benefit severe asthma, although specific data in this group are scarce since many studies focus on mild to moderate disease. People with severe disease may actually show greater improvement with interventions,39 given their poorer HRQoL and, as others argue,44,45 greater motivation to engage with interventions likely to improve HRQoL. The most consistent evidence for improving HRQoL in moderate to severe asthma is for self-management education (standardised mean difference [SMD] = 0.29; 95% confidence interval [CI], 0.11–0.47).37,39 More intensive self-management interventions, such as structured chronic disease management programs, appear to have the greatest impact (SMD = 0.22; 95% CI, 0.08–0.37),38,46 compared with more minimal interventions, such as the provision of personalised written action plans alone. Although personalised written action plans help to improve asthma control in adults and children with asthma, their impact on overall HRQoL is small (SMD = 0.18; 95% CI, 0.05–0.30).47,48 Mental health problems, obesity and physical inactivity are associated with substantial deficits in HRQoL; therefore, addressing these with evidence-based approaches is also warranted.30,49,50 Importantly, non-pharmacological interventions may have an impact across multiple quality of life domains, risk factors and comorbidities. For instance, exercise intervention not only reduces physical inactivity, it can also improve obesity, lung function and mental health41,42,51 and result in clinically significant improvements in HRQoL (65% achieving minimally clinically significant improvement v 40% in the control42).
Pharmacological Interventions and health-related quality of life
Established asthma therapies
Novel and established medications designed to treat severe asthma will only be covered briefly in this article as they have been reviewed in detail elsewhere in this issue.52 A systematic review examining HRQoL in relation to asthma medication concluded that all medication reviewed, with the exception of short-acting β-agonists, improved HRQoL among adults and adolescents with persistent, symptomatic and/or uncontrolled asthma.53 The medications evaluated included inhaled corticosteroids (ICS), long-acting β-agonists (LABA), combination therapies (ICS/LABA), leukotriene receptor antagonists and theophylline.53 The largest improvements in the Asthma Quality of Life Questionnaire (www.qoltech.co.uk/aqlq.html) were noted for combination therapies (ICS/LABA). Additionally, a review and meta-analysis53 noted a large placebo effect for the non-intervention arms of the reviewed drug trials. This review, however, did not have a specific severe asthma focus,53 where HRQoL is more significantly impaired.54
Over 25% of people with severe asthma take maintenance oral corticosteroids.36 This corticosteroid-dependent group have poorer HRQoL and more comorbidities than those with severe asthma who are not corticosteroid-dependent.55 These patients report many adverse side effects from oral corticosteroids, including depression, sleep problems, weight gain, irritability and mood swings.33
Novel asthma therapies
Novel pharmacological therapies have been developed to target specific phenotypes or biological pathways in severe asthma.1,56 Monoclonal antibody therapies have been trialled for type 2 inflammation-high asthma, a major subset of asthma characterised by airway and blood eosinophilia driven by aberrant production of the type 2 cytokines interleukin (IL)-5, IL-13 and IL-4. These type 2 targeted therapies have had positive effects on reducing severe attacks.57 Targeted therapies include IL-5 signalling antagonists (mepolizumab, reslizumab and benralizumab), IgE targeting (omalizumab), IL-4 and IL-13 signalling (dupilumab), and thymic stromal lymphopoietin targeting (tezepelumab58) monoclonal antibody therapy. Tezepelumab has also shown favourable results for non-type 2 asthma.58 In addition to monoclonal antibody therapies, there has also been increased use of maintenance macrolide antibiotics as add-on therapies in people with moderate to severe asthma, with one study reporting a 40% reduction in severe attacks in this population.1 Novel monoclonal antibody therapies have been shown to improve HRQoL; however, despite these improvements, no studies demonstrate a magnitude that surpasses the minimal clinically important difference when compared with the placebo arm, and patients continue to experience HRQoL impairment.53,58-63
Measurement of quality of life
HRQoL is an independent predictor of clinical outcomes in asthma, such as exacerbations, urgent doctor visits and presentations to emergency departments.64-66 Therefore, its valid and accurate measurement is crucial to ensuring that treatments meaningfully improve patients’ lives and clinical outcomes. Determining whether treatment has had a meaningful impact on HRQoL in the clinical setting requires measurement with reliable and valid tools. Patient-reported outcome measures are self-report questionnaires completed by patients that measure symptoms, functional and health status, and social and psychological wellbeing (an extensive list of validated measures can be found in the Severe Asthma Toolkit, https://toolkit.severeasthma.org.au/resources/asthma-assessment-resources). In practice, these are infrequently used in severe asthma, largely due to their length, difficult administration and complex scoring structures, rendering them impractical in the clinical setting.67,68
Furthermore, a key recommendation of the Food and Drug Administration is that patient-reported outcome measures be fit for purpose,69 but none of the currently available asthma HRQoL measures have been developed specifically in a severe asthma population. This highlights a need for the development of fit-for-purpose severe asthma measures that can be easily implemented in the clinic.
Patients value their relationship with health care professionals, and people with severe asthma express a preference for a partnership in terms of decision making and a need to “feel heard”;13 in the absence of this, patients may intentionally not adhere to their doctors’ instructions. This is highlighted in a qualitative study,70 where a 31-year-old woman states: “I didn’t follow [the action plan] because I never got to express my opinion. A lot of the doctors that I have come across don’t allow you to express your opinion at all. That really does not work because they have stronger opinions. So … listening to the patient as opposed to a closed conversation”. The time spent with health care professionals is also important; feeling like the doctor has sufficient time to discuss their problems made patients feel more valued.70
Therefore, in the absence of severe asthma-specific HRQoL measures that can be easily used in clinical practice, a consideration of what is important to the patient may improve the clinician’s understanding of quality of life. In a randomised controlled trial,71 patients with poorly controlled asthma were randomly allocated to shared decision making or clinician decision making. In the shared decision making group, treatment was negotiated with the participants, who had the opportunity to summarise their treatment goals, were provided with information about the necessary treatments for disease control, and were presented with a range of treatment options which enabled them to make a shared decision with the clinician. This approach resulted in statistically significant improvements in the primary outcome of medication adherence and clinically significant improvements in quality of life compared with those in the clinician decision making group.71
Conclusion
While novel targeted treatments have indeed had an impact on HRQoL for people with severe asthma, patients continue to experience an excessive burden on their physical, emotional and social functioning. Future research is required focusing on innovative pharmacological treatment interventions that specifically consider the severe asthma population. In addition, non-pharmacological and behavioural interventions need to be developed for and tested in populations with severe asthma to examine their impact on HRQoL. Evaluation of multidimensional interventions that target the wide range of disease traits and comorbidities are needed. In order to effectively assess the impact of these interventions on HRQoL, we need patient-reported outcome measures that are developed and validated specifically for people with severe asthma.
Box 1 – Quality of life impacts and practical guide to assessment and intervention
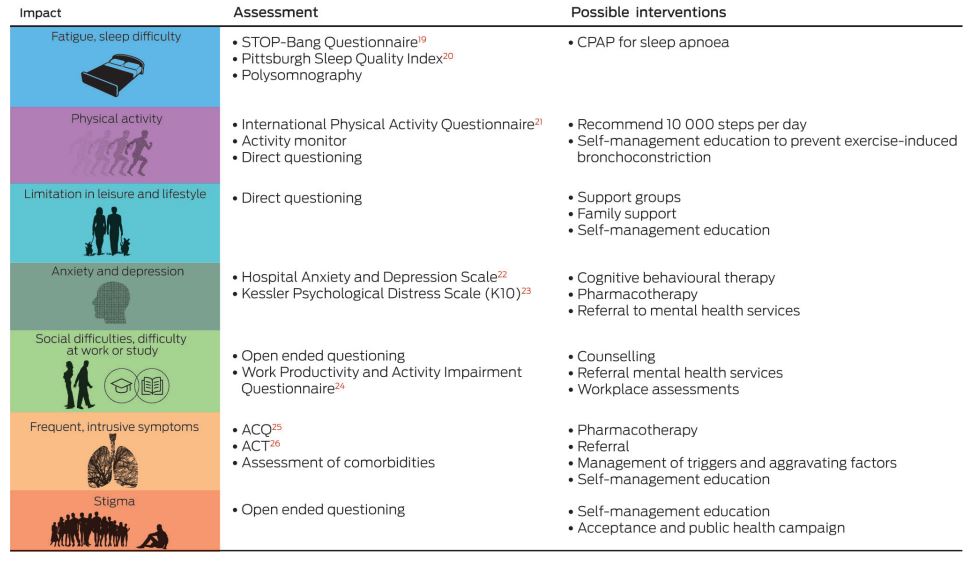
ACQ = Asthma Control Questionnaire. ACT = Asthma Control Test. CPAP = continuous positive airway pressure. For further information, see the Severe Asthma Toolkit: https://toolkit.severeasthma.org.au.
Box 2 – Effects of multidimensional assessment and treatment based on a meta-analysis of three before and after studies (n = 451 participants)36*
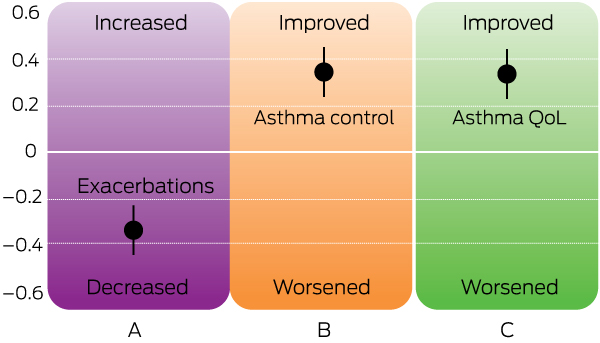
QoL = quality of life. * Bars represent standardised mean difference with 95% confidence intervals for exacerbations (A), asthma control (Asthma Control Questionnaire or Asthma Control Test) (B), and asthma-related quality of life (Asthma Quality of Life Questionnaire) (C), before and after multidimensional assessment and treatment. Positive effect size indicates an improvement in asthma control or asthma-related quality of life; negative effect size indicates a reduction in exacerbations. Source: The image has been adapted, with permission, from the Centre of Excellence in Severe Asthma.36
Provenance: Commissioned; externally peer reviewed.
- Vanessa M McDonald1,2
- Sarah A Hiles1,2
- Kimberley A Jones1,2
- Vanessa L Clark1,2
- Janelle Yorke3
- 1 Priority Research Centre for Healthy Lungs, University of Newcastle, Newcastle, NSW
- 2 Centre of Excellence in Severe Asthma, University of Newcastle, Newcastle, NSW
- 3 University of Manchester, Manchester, UK
Vanessa Clark receives a fellowship from the National Health and Medical Research Council Centre of Excellence in Severe Asthma. Vanessa McDonald is supported by an NHMRC TRIP fellowship.
Vanessa McDonald has received speaker fees for unrelated work from AstraZeneca, grants for organising education unrelated to this work from Menarini, and research funds for unrelated work from AstraZeneca and GlaxoSmithKline. Sarah Hiles was supported by research funds from GlaxoSmithKline and AstraZeneca during the conduct of the review.
- 1. McDonald VM, Maltby S, Reddel HK, et al. Severe asthma: current management, targeted therapies and future directions — a roundtable report. Respirology 2017; 22: 53-60.
- 2. Dean BB, Calimlim BC, Sacco P, et al. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes 2010; 8: 1-10.
- 3. Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/2020/about/foundation-health-measures/Health-Related-Quality-of-Life-and-Well-Being (viewed Mar 2018).
- 4. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765.
- 5. Fleming L, Murray C, Bansal AT, et al; U-BIOPRED Study Group. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46: 1322-1333.
- 6. Nordlund B, Konradsen JR, Pedroletti C, et al. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr 2011; 100: 1454-1460.
- 7. Shaw DE, Sousa AR, Fowler SJ, et al; U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46: 1308-1321.
- 8. Dockrell M, Partridge MR, Valovirta E. The limitations of severe asthma: the results of a European survey. Allergy 2007; 62: 134-141.
- 9. Nelsen LM, Kimel M, Murray LT, et al. Qualitative evaluation of the St George’s Respiratory Questionnaire in patients with severe asthma. Respir Med 2017; 126: 32-38.
- 10. Aburuz S, Gamble J, Heaney LG. Assessment of impairment in health-related quality of life in patients with difficult asthma: psychometric performance of the Asthma Quality of Life Questionnaire. Respirology 2007; 12: 227-233.
- 11. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007; 16: 22-27.
- 12. Wenzel SE, Brillhart S, Nowack K. An invisible disease: severe asthma is more than just “bad asthma”. Eur Respir J 2017; 50: 1701109.
- 13. Eassey D, Reddel HK, Foster JM, et al. “… I’ve said I wish I was dead, you’d be better off without me”: a systematic review of people’s experiences of living with severe asthma. J Asthma 2018: 1-12.
- 14. McDonald VM, Yorke J. Adherence in severe asthma: time to get it right. Eur Respir J 2017; 50: 1702191.
- 15. Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J 2018; 51: 1701836.
- 16. Hiles SA, Harvey ES, McDonald VM, et al. Working while unwell: workplace impairment in people with severe asthma. Clin Exp Allergy 2018;48: 650-662.
- 17. Porsbjerg C, Rasmussen L, Nolte H, Backer V. Association of airway hyperresponsiveness with reduced quality of life in patients with moderate to severe asthma. Ann Allergy Asthma Immunol 2007; 98: 44-50.
- 18. Bardin PG, Rangaswamy J, Yo SW. Managing comorbid conditions in severe asthma. Med J Aust 2018; 209 (2 Suppl): S11-S17.
- 19. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149: 631-638.
- 20. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193-213.
- 21. Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381-1395.
- 22. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361-370.
- 23. Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002; 32: 959-976.
- 24. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of aWork Productivity and Activity Impairment instrument. Pharmacoeconomics 1993; 4: 353-365.
- 25. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the Asthma Control Questionnaire. Respir Med 2005; 99: 553-558.
- 26. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol 2004; 113: 59-65.
- 27. Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med 2009; 180: 817-822.
- 28. Chen H, Blanc PD, Hayden ML, et al; TENOR Study Group. Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health 2008; 11: 231-239.
- 29. Haselkorn T, Chen H, Miller DP, et al. Asthma control and activity limitations: insights from the Real-world Evaluation of Asthma Control and Treatment (REACT) Study. Ann Allergy Asthma Immunol 2010; 104: 471-477.
- 30. Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol Pract 2017; 6: 814-822.
- 31. Franco R, Nascimento HF, Cruz AA, et al. The economic impact of severe asthma to low-income families. Allergy 2009; 64: 478-483.
- 32. McDonald VM, Godbout K, Hiles S, et al; SAWD Investigators. Severe asthma treatable traits: prevalence and exacerbation prediction. Respirology 2018; 23 (Suppl 1): 21-103; TO 091.
- 33. Hyland ME, Whalley B, Jones RC, Masoli M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res 2015; 24: 631-639.
- 34. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343-373.
- 35. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet 2010; 376: 803-813.
- 36. Clark VL, Gibson PG, Genn G, et al. Multidimensional assessment of severe asthma: a systematic review and meta-analysis. Respirology 2017; 22: 1262-1275.
- 37. Gibson PG, Powell H, Wilson A, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2002: CD001117.
- 38. Peytremann-Bridevaux I, Arditi C, Gex G, et al. Chronic disease management programmes for adults with asthma. Cochrane Database Syst Rev 2015: CD007988.
- 39. Wolf F, Guevara JP, Grum CM, et al. Educational interventions for asthma in children. Cochrane Database Syst Rev 2002: CD000326.
- 40. Kew KM, Nashed M, Dulay V, Yorke J. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev 2016: CD011818.
- 41. Dias-Júnior SA, Reis M, de Carvalho-Pinto RM, et al. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J 2014; 43: 1368-1377.
- 42. Freitas PD, Ferreira PG, Silva AG, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 32-42.
- 43. Nyenhuis SM, Dixon AE, Ma J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: what is the evidence? J Allergy Clin Immunol Pract 2017; 6: 751-763.
- 44. Marcano Belisario JS, Huckvale K, Greenfield G, et al. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev 2013: CD010013.
- 45. Scott HA, Gibson PG, Garg ML, et al. Determinants of weight loss success utilizing a meal replacement plan and/or exercise, in overweight and obese adults with asthma. Respirology 2015; 20: 243-250.
- 46. Verkleij M, Beelen A, van Ewijk BE, Geenen R. Multidisciplinary treatment in children with problematic severe asthma: a prospective evaluation. Pediatr Pulmonol 2017; 52: 588-597.
- 47. Bhogal SK, Zemek RL, Ducharme F. Written action plans for asthma in children. Cochrane Database Syst Rev 2006: CD005306.
- 48. Gatheral TL, Rushton A, Evans DJ, et al. Personalised asthma action plans for adults with asthma. Cochrane Database Syst Rev 2017: CD011859.
- 49. Strine TW, Mokdad AH, Balluz LS, et al. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J Asthma 2008; 45: 123-133.
- 50. Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016; 21: 1384-1390.
- 51. França-Pinto A, Mendes FAR, de Carvalho-Pinto RM, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax 2015; 70: 732-739.
- 52. Upham JW, Chung LP. Optimising treatment for severe asthma. Med J Aust 2018; 209 (2 Suppl): S22-S27.
- 53. Bateman ED, Esser D, Chirila C, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol 2015; 136: 914-922.
- 54. Hossny E, Caraballo L, Casale T, et al. Severe asthma and quality of life. World Allergy Organ J 2017; 10: 28.
- 55. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016; 71: 339-346.
- 56. Grainge CL, Maltby S, Gibson PG, et al. Targeted therapeutics for severe refractory asthma: monoclonal antibodies. Expert Rev Clin Pharmacol 2016; 9: 927-941.
- 57. Fricker M, Heaney LG, Upham JW. Can biomarkers help us hit targets in difficult-to-treat asthma? Respirology 2017; 22: 430-442.
- 58. Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936-946.
- 59. Bleecker ER, FitzGerald JM, Chanez P, et al; SIROCCO Study Investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115-2127.
- 60. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355-366.
- 61. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659-668.
- 62. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet; 380: 651-659.
- 63. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31-44.
- 64. Magid DJ, Houry D, Ellis J, et al. Health-related quality of life predicts emergency department utilization for patients with asthma. Ann Emerg Med; 43: 551-557.
- 65. Mancuso CA, Rincon M, McCulloch CE, Charlson ME. Self-efficacy, depressive symptoms, and patients’ expectations predict outcomes in asthma. Med Care 2001; 39: 1326-1338.
- 66. Schatz M, Zeiger RS, Mosen D, Vollmer WM. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care 2008; 14: 206-211.
- 67. Wilson SR, Rand CS, Cabana MD, et al. Asthma outcomes: quality of Life. J Allergy Clin Immunol 2012; 129: S88-S123.
- 68. Win T, Pearce L, Nathan J, et al. Use of the Airway Questionnaire 20 to detect changes in quality of life in asthmatic patients and its association with the St George’s Respiratory Questionnaire and clinical parameters. Can Respir J 2008; 15: 133-137.
- 69. US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4: 79.
- 70. Ross CJ, Williams BA, Low G, Vethanayagam D. Perceptions about self-management among people with severe asthma. J Asthma 2010; 47: 330-336.
- 71. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010; 181: 566-577.





Summary