The known Oxygen therapy increases Paco2 in patients with conditions associated with chronic respiratory failure, such as chronic obstructive pulmonary disease and obesity hypoventilation syndrome.
The new High concentration oxygen therapy increased Ptco2 in morbidly obese patients when compared with titrated oxygen therapy with a target Spo2 of 88–92%.
The implications Our findings support guidelines that advocate employing titrated oxygen therapy to achieve a target Spo2 of 88–92% if oxygen therapy is required by patients with morbid obesity.
Oxygen therapy increases the arterial partial pressure of carbon dioxide (Paco2) in patients with stable and acute exacerbations of chronic obstructive pulmonary disease (COPD).1,2 The hypercapnia and associated respiratory acidosis can be marked, and contribute to mortality being more than twice as high among patients with acute exacerbations of COPD who receive high concentration oxygen therapy than among those for whom oxygen administration is titrated to achieve a target oxygen saturation of 88–92%, as measured by pulse oximetry (peripheral oxygen saturation, Spo2).3
There is also evidence that administering high concentration oxygen may increase Paco2 in patients with other respiratory conditions, such as acute asthma,4-6 community-acquired pneumonia,7 and stable obesity hypoventilation syndrome (OHS).8-12 Guidelines therefore recommend that oxygen therapy be titrated to a target Spo2 range to avoid the risks of both hypoxaemia and hyperoxaemia.13,14
In our earlier randomised crossover trial of the effects of 100% oxygen therapy in patients with OHS, three of 24 participants were withdrawn early because Paco2 increased by more than 10 mmHg in less than 20 minutes, indicating that high concentration oxygen administration can result in rapid, clinically significant increases in the Paco2.8 Patients at greatest risk of worsening hypercapnia were those with the most marked hypoxaemia; that is, those most likely to receive oxygen therapy. The generalisability of our findings to patients with morbid obesity was limited by the requirement that participants had chronic respiratory failure, in accordance with the definition of OHS.15 Generalisability to clinical practice was also limited by administering 100% oxygen for 20 minutes rather than lower concentrations for longer periods, which is more likely in clinical settings.
The aim of our study was to compare the effects on Paco2 of high concentration and titrated oxygen in medical inpatients with morbid obesity who were not selected for a pre-existing diagnosis of OHS. To assess the physiological response, we continuously monitored transcutaneous carbon dioxide tension (Ptco2), a non-invasive validated measure of Paco2.16-18 Our hypothesis was that high concentration oxygen therapy would increase Ptco2 to a significantly greater extent than an oxygen regimen in which administration, if required, is titrated to achieve an Spo2 target of 88–92%, as recommended when treating patients with conditions associated with chronic respiratory failure.13,14
Methods
For this randomised crossover trial, we recruited 24 adult patients with a body mass index (BMI) exceeding 40 kg/m2 who had been admitted to Wellington Regional Hospital, New Zealand. Patients were excluded if they were under 16 years of age, had been diagnosed with COPD or a condition associated with restriction of chest wall expansion other than obesity, unstable angina or recent myocardial infarction, had a baseline Ptco2 greater than 65 mmHg, or were currently receiving non-invasive ventilation. Investigators could also exclude patients for any condition that might represent a safety risk or affect the feasibility of the study or its results.
After written informed consent had been provided, demographic and clinical data were collected. Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines19 with a handheld spirometer (CareFusion). Transcutaneous monitors on the earlobe measured Spo2, Ptco2 and heart rate. Measurements for the first 12 participants were recorded with the TOSCA 500 monitor (Radiometer); as this was not available for the second 12, their data were collected with a SenTec monitor (SenTec). Respiratory rate was measured at each time point over 60 seconds by direct observation.
Participants were randomised to the order in which they received 60 minutes of titrated oxygen therapy (oxygen delivered, if required, via nasal prongs to achieve Spo2 of 88–92%) or high concentration oxygen therapy (delivered via Hudson mask at 8 L/min, without regard to Spo2). The randomisation schedule was generated by the study statistician; the intervention order for each participant was sealed in an opaque envelope and opened by the study investigator at randomisation. The washout period between the two interventions was at least 30 minutes. If participants were receiving oxygen at the start of the study, it was titrated for 30 minutes to achieve an Spo2 of 88–92% before the first intervention, and for a minimum of 30 minutes during the washout period before the second intervention; this was undertaken to standardise baseline conditions. Ptco2, Spo2, respiratory rate, and heart rate were recorded at baseline, and every 10 minutes during the interventions. Ptco2 was monitored continuously; if it increased by more than 10 mmHg from baseline, the intervention was ended.
The primary outcome variable was Ptco2 at 60 minutes, adjusted for baseline. Secondary outcome variables included the proportion of participants at each time point with a change in Ptco2 from baseline of least 4 mmHg; the proportion with this change at at least one time point; and Ptco2, Spo2, heart rate, and respiratory rate at all time points, adjusted for baseline. Differences in outcome variables according to treatment were estimated by mixed linear models with fixed effects for all the baseline measurements of the particular outcome variables, intervention order, and intervention. A random effect for participants accounted for the crossover design. These estimates are presented as differences between the high concentration oxygen and titrated oxygen interventions, with 95% confidence intervals (CIs). The difference (with 95% CI) in the proportion of participants with a Ptco2 rise of at least 4 mmHg was assessed in a McNemar test. Whether the change in monitor affected the differences between randomised treatments was explored by post hoc addition of fixed effects for a main effect and an interaction effect. In a further simple post hoc analysis, the difference between the oxygen intervention types in two subgroups was compared in t tests: participants who had a baseline Ptco2 greater than 45 mmHg, and those admitted with a respiratory diagnosis.
All analyses were conducted in SAS 9.3 (SAS Institute). A sample size of 24 was calculated to have 80% power (α = 0.05) to detect a difference in Ptco2 of 2.4 mmHg between the groups, half the mean difference found by a study of participants with OHS (5 mmHg; standard deviation, 4 mmHg).8
Ethics approval
The study was prospectively approved by the New Zealand Health and Disability Ethics Committee – Central (reference, CEN/10/03/08) and was registered with the trial protocol with the Australian New Zealand Clinical Trials Registry (ACTRN12610000522011).
Results
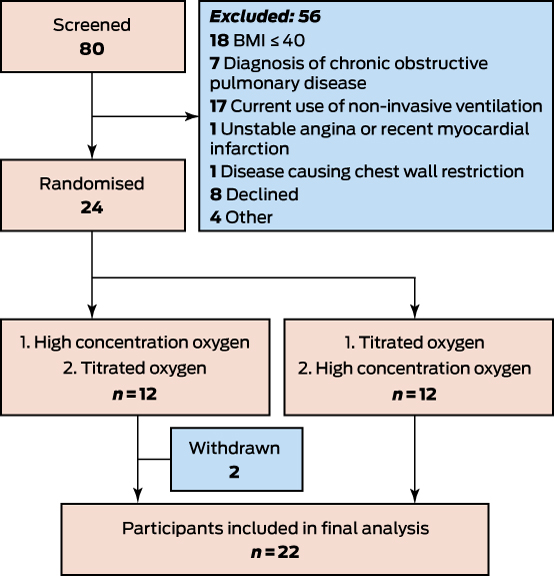
Participants were recruited during February–September 2015. Eighty inpatients were screened, and 24 were randomised to treatment (Box 1). One participant withdrew after randomisation when given the opportunity to be discharged home, and one participant was randomised in error a second time during a subsequent admission; both were excluded from the analysis. The remaining 22 patients were studied at a median 0.9 days (range, 0.2–6.5 days) after admission.
The baseline characteristics of the participants are summarised in Box 2. The median Spo2 was 97% (range, 89–100%); the mean Ptco2 was 41.7 mmHg, and 16 participants (73%) had a baseline Ptco2 of 45 mmHg or less. No participant had a previous diagnosis of OHS; six participants reported a history of witnessed apnoea and snoring, but none had been diagnosed with obstructive sleep apnoea. Seven participants had an admission diagnosis of asthma, and two were admitted with a lower respiratory tract infection. Three participants (two with asthma, one with heart failure) required up to 1 L/min oxygen via nasal cannulae during the titration intervention to maintain Spo2 at 88–92%.
All 22 participants completed both interventions, although high concentration oxygen was interrupted for 3 minutes for one participant at 32 minutes while they visited the bathroom. The baseline Ptco2 values for all but two participants were within 4 mmHg of each other for both interventions; the mean baseline Ptco2 before intervention was 41.8 mmHg for titrated oxygen and 41.7 mmHg for high flow oxygen. The effect of randomisation order was not statistically significant for any of the Ptco2 analyses.
The mean difference in Ptco2 at 60 minutes, adjusted for baseline, between the high concentration oxygen and titrated oxygen interventions was 3.2 mmHg (95% CI, 1.3–5.2; P = 0.002) (Box 3). The increase in Ptco2 with high concentration oxygen therapy and the difference between the two interventions were evident as early as 10 minutes after commencing the interventions (online Appendix, tables 1, 2). The difference in Ptco2 at 60 minutes was greater for participants with a baseline Ptco2 exceeding 45 mmHg (P = 0.043) (Box 4), but admission diagnosis (respiratory v non-respiratory) did not affect this outcome (Box 5).
The increase in Ptco2 from baseline was 4 mmHg or more for 14 participants during the high concentration intervention and for two during the titrated intervention (online Appendix, table 3). The proportion of participants in whom Ptco2 increased by 4 mmHg or more from baseline was significantly greater for the high concentration intervention than for the titration intervention (online Appendix, table 4). For one participant, an increase in Ptco2 of 9.7 mmHg during the high concentration intervention was measured during continuous monitoring; they were sleepy but rousable. For nine of the 14 participants in whom Ptco2 increased by at least 4 mmHg over baseline with high concentration oxygen, baseline Ptco2 had been below 45 mmHg. For one participant, Ptco2 was reduced by 13 mmHg at 50 minutes and by 14 mmHg at 60 minutes during the titrated intervention, associated with movement to a more upright position; Ptco2 was not reduced by 4 mmHg or more from baseline for any other participant during either intervention.
There was no interaction between treatment and transcutaneous monitor type (for interaction, P = 0.50). In a model that incorporated a fixed effect for the monitor type, the estimate of the differences between titrated and high concentration oxygen was the same (data not shown).
The change in Spo2 from baseline was significantly greater at all time points for high concentration oxygen than for titrated oxygen treatment. The change in heart rate was significantly lower with high concentration oxygen at most time points. The respiratory rate did not differ between the two interventions (online Appendix, tables 1, 2).
Discussion
We found that high concentration oxygen therapy increased Ptco2 in morbidly obese hospital inpatients to a significantly greater degree than titrating oxygen to achieve a target Spo2 of 88–92%, regardless of whether hypercapnia was present at baseline or the patient had an acute respiratory disorder. These observations support guidelines that recommend titrated oxygen rather than unrestricted high concentration therapy in patients with conditions associated with chronic respiratory failure, such as morbid obesity13 and OHS.14 Further, these results build on earlier observations that oxygen increases Ptco2 in patients with OHS8,12 by reporting a similar physiological response in patients with simple morbid obesity, without a diagnosis of OHS. About two-thirds of the participants with rises in Ptco2 of at least 4 mmHg were not hypercapnic at baseline, indicating that hypercapnia is not a prerequisite for oxygen-induced elevations in Paco2 in patients with obesity, as is also the case for patients with respiratory conditions.1,4-7
For the high concentration oxygen intervention, we administered oxygen at 8 L/min through a Hudson mask. It is reasonable to assume that such a regimen may be employed in an acute medical setting in breathless, morbidly obese patients, regardless of the presence of hypoxaemia; it has been reported that high concentration oxygen is commonly administered to COPD patients without evidence of hypoxaemia.20-22
The mean increase in Ptco2 of 3.2 mmHg after 8 L/min oxygen through a Hudson mask (equivalent to a fraction of inspired oxygen [Fio2] of at least 0.5)23 we measured was similar to the 3.8 mmHg increase in capillary partial pressure of carbon dioxide (Pcco2) in the study by Hollier and colleagues, in which an Fio2 of 0.50 was administered to stable OHS patients.12 The authors reported that this level of Pcco2 increase was associated with a fall in blood pH from 7.373 to 7.346,12 resulting in respiratory acidosis likely to be clinically significant; pH below 7.35 is associated with increased mortality and a greater need for intubation in patients with COPD.24,25 Importantly, the severity of an exacerbation is indicated not by the absolute level of hypercapnia, but rather by the increase above the chronic stable Paco2 value, as reflected by increased acidosis.24 In the Hollier study, an Fio2 of 0.28 increased Pcco2 by 2.3 mmHg and an Fio2 of 0.50 increased Pcco2 by 3.8 mmHg in patients with OHS respectively,12 whereas in our earlier study an Fio2 of 1.0 in OHS patients increased Ptco2 by 5.0 mmHg.8 Recognising that both Ptco216-18 and Pcco226 are validated indirect measures of Paco2, this dose–response relationship between Paco2 and Fio2 supports the use of titrated oxygen, with reduced exposure to higher oxygen concentrations reducing the risk of oxygen-induced hypercapnia.
The physiological response to high concentration oxygen was variable, with an increase in Ptco2 of at least 8 mmHg from baseline in three of 22 participants during 60 minutes of oxygen therapy. Such variability has been noted previously,8-12 suggesting it might be clinically useful to be able to identify adverse responders. The rise in Ptco2 with high concentration oxygen therapy was greater in those with hypercapnia at baseline, indicating that those with chronic respiratory failure were at greater risk of life-threatening hypercapnia during oxygen therapy, as reported earlier.12 This is relevant, as about one-third of patients with morbid obesity also have chronic respiratory failure.8,15
The characteristics of the participants are relevant to the external validity of our findings. Our participants were morbidly obese, with a mean BMI of 50.4 kg/m2. None had been diagnosed with OHS, and only six had baseline Ptco2 values greater than 45 mmHg, indicating that most participants did not have chronic respiratory failure at baseline. This proportion is similar to the one in three patients with morbid obesity in whom awake hypercapnia was observed during screening for our previous study,8 and with estimates of the prevalence of OHS among hospitalised obese inpatients.15 The comorbidities reflect the range of acute medical conditions of patients admitted to internal medicine departments, apart from COPD. Seven participants had an admission diagnosis of acute exacerbation of asthma, and two had lower respiratory tract infections. While Ptco2 may rise in patients with these acute respiratory conditions during oxygen therapy,4-7 whether participants were admitted with a respiratory diagnosis had no effect on the magnitude of the Ptco2 change in our study.
We used Ptco2 as a painless, non-invasive and validated measure for continuously monitoring changes in Paco2 in response to oxygen,4,7,8,17,18 and which can be used to accurately estimate changes in Pcco2 from baseline.16 Neither arterial nor capillary blood gas sampling was attempted, as these invasive methods do not allow continuous assessment of Pco2. The Ptco2 monitor employed (TOSCA or SenTec) had no effect on the observed differences between the two treatments.
We employed a crossover design, with adequate washout between interventions.27 The nature of the interventions did not allow blinding of investigators or participants. The study was not designed to assess why high concentration oxygen increased Ptco2 in people with morbid obesity; reduced minute ventilation,8 reduced tidal volume,12 and absorption atelectasis10 have been proposed as possible mechanisms.
In conclusion, we found that high concentration oxygen therapy increases Ptco2 in patients with morbid obesity with or without hypercapnia at baseline. This finding extends previous observations that this response is seen in a range of respiratory conditions, including COPD,1-3 asthma,4-6 pneumonia,7 and OHS.8,12 The effect of oxygen therapy in patients with other conditions associated with chronic respiratory failure should be investigated, including bronchiectasis and neuromuscular disease, for which high quality evidence is lacking. Our findings support guideline recommendations that oxygen be titrated to a target Spo2 of 88–92% in patients with conditions associated with hypercapnic respiratory failure, such as morbid obesity.13,14
Box 2 – Baseline characteristics of the participants
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Age (years), mean (SD) |
53.5 (16.1) |
||||||||||||||
|
BMI (kg/m2), mean (SD) |
50.4 (9.2) |
||||||||||||||
|
FEV1/FVC (n = 19), mean (SD) |
0.75 (0.08) |
||||||||||||||
|
Ptco2 (mmHg), mean (SD) |
41.7 (5.8) |
||||||||||||||
|
Oxygen saturation (%), mean (SD) |
96.1 (2.6) |
||||||||||||||
|
Sex (male) |
7 (32%) |
||||||||||||||
|
Ethnic background |
|
||||||||||||||
|
Māori |
3 (14%) |
||||||||||||||
|
New Zealand European |
8 (36%) |
||||||||||||||
|
Pacific Islander |
11 (50%) |
||||||||||||||
|
Time since admission (days) |
|
||||||||||||||
|
1 |
13 (59%) |
||||||||||||||
|
2 |
5 (23%) |
||||||||||||||
|
3 or more |
4 (18%) |
||||||||||||||
|
Diagnosis |
|
||||||||||||||
|
Cellulitis |
6 (27%) |
||||||||||||||
|
Asthma exacerbation |
7 (32%) |
||||||||||||||
|
Lower respiratory tract infection |
2 (9%) |
||||||||||||||
|
Other |
7 (32%) |
||||||||||||||
|
Smoking status |
|
||||||||||||||
|
Current |
1 (4%) |
||||||||||||||
|
Ex-smoker |
12 (54%) |
||||||||||||||
|
Never smoked |
9 (41%) |
||||||||||||||
|
|
|||||||||||||||
|
BMI = body mass index; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; Ptco2 = transcutaneous carbon dioxide tension. |
|||||||||||||||
Box 3 – Differences in outcomes at 60 minutes between the high concentration and titrated oxygen interventions, adjusted for baseline
|
|
High concentration oxygen (A) |
Titrated oxygen (B) |
Difference in outcome (A – B) (95% CI) |
P |
|||||||||||
|
Baseline |
60 minutes |
Baseline |
60 minutes |
||||||||||||
|
|
|||||||||||||||
|
Ptco2 (mmHg), mean (SD) |
41.7 (5.7) |
44.4 (7.1) |
41.8 (5.9) |
41.3 (6.2) |
3.2 (1.3 to 5.2) |
0.002 |
|||||||||
|
Respiratory rate (breaths per minute), mean (SD) |
18.3 (4.3) |
19.8 (3.5) |
18.0 (3.5) |
20.4 (3.5) |
–0.7 (–2.3 to 0.9) |
0.38 |
|||||||||
|
Heart rate (beats per minute), mean (SD) |
85.8 (14.4) |
83.4 (14.8) |
85.8 (15.4) |
88.3 (14.7) |
–4.8 (–8.5 to –1.2) |
0.010 |
|||||||||
|
Oxygen saturation (%), mean (SD) |
96.2 (2.5)* |
99.5 (0.9) |
96.0 (3.1) |
95.9 (2.9) |
3.4 (2.6 to 4.3) |
< 0.001 |
|||||||||
|
|
|||||||||||||||
|
Ptco2 = transcutaneous carbon dioxide tension. * n = 21 because of an error in recording baseline data. |
|||||||||||||||
Box 4 – Change in transcutaneous carbon dioxide tension (Ptco2) from baseline at 60 minutes, by baseline Ptco2
|
|
Baseline Ptco2 |
Difference in outcome (A – B) (95% CI) |
P |
||||||||||||
|
≤ 45 mmHg (A) |
> 45 mmHg (B) |
||||||||||||||
|
|
|||||||||||||||
|
Number of participants |
16 |
6 |
|
|
|||||||||||
|
High concentration oxygen, mean change (SD) |
2.4 (2.7) |
3.8 (1.7) |
–1.4 (–3.9 to 1.0) |
0.24 |
|||||||||||
|
Titrated oxygen, mean change (SD) |
0.2 (2.0) |
–2.4 (6.2) |
2.7 (–3.8 to 9.1) |
0.13 |
|||||||||||
|
Difference between high concentration and titrated oxygen therapy, mean (SD) |
2.1 (3.3) |
6.2 (5.6) |
–4.1 (–8.1 to –0.1) |
0.043 |
|||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 5 – Change in transcutaneous carbon dioxide tension (Ptco2) from baseline at 60 minutes, by admission diagnosis
|
|
Admission diagnosis |
Difference (A – B) |
P |
||||||||||||
|
Respiratory disorder (A) |
Other (B) |
||||||||||||||
|
|
|||||||||||||||
|
Number of participants |
9 |
13 |
|
|
|||||||||||
|
High concentration oxygen, mean change (SD) |
2.6 (2.3) |
2.8 (2.7) |
–0.2 (–2.5 to 2.1) |
0.85 |
|||||||||||
|
Titrated oxygen, mean change (SD) |
–1.2 (1.8) |
0.0 (4.5) |
–1.2 (–4.5 to 2.1) |
0.46 |
|||||||||||
|
Difference between high concentration and titrated oxygen therapy, mean (SD) |
3.8 (3.1) |
2.8 (5.1) |
1.0 (–3.0 to 5.0) |
0.61 |
|||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 20 March 2017, accepted 18 July 2017
- Janine Pilcher1
- Michael Richards1
- Leonie Eastlake1
- Steven J McKinstry1
- George Bardsley1
- Sarah Jefferies1
- Irene Braithwaite1
- Mark Weatherall2
- Richard Beasley1
- 1 Medical Research Institute of New Zealand, Wellington, New Zealand
- 2 Wellington School of Medicine, University of Otago, Wellington, New Zealand
This study was funded by the Health Research Council of New Zealand (HRC). Janine Pilcher and Irene Braithwaite received HRC clinical training fellowships (12/879, 14/040). The Medical Research Institute of New Zealand receives funding from the HRC Independent Research Organisations Capability Fund (14/1002). The HRC had no involvement in the design of the study, collection, analysis or interpretation of the data, nor in the decision to submit the results for publication.
Janine Pilcher, Michael Richards, Leonie Eastlake and Richard Beasley are members of the Thoracic Society of Australia and New Zealand Adult Oxygen Guidelines Group. Richard Beasley is a member of the BTS Emergency Oxygen Guideline Group.
- 1. Murphy R, Driscoll P, O’Driscoll R. Emergency oxygen therapy for the COPD patient. Emerg Med J 2001; 18: 333-339.
- 2. Pilcher J, Weatherall M, Perrin K, Beasley R. Oxygen therapy in acute exacerbations of COPD. Expert Rev Respir Med 2015; 9: 287-293.
- 3. Austin MA, Wills KE, Blizzard L, et al. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 2010; 341: c5462.
- 4. Perrin K, Wijesinghe M, Healy B, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax 2011; 66: 937-941.
- 5. Chien JW, Ciufo R, Novak R, et al. Uncontrolled oxygen administration and respiratory failure in acute asthma. Chest 2000; 117: 728-733.
- 6. Rodrigo GJ, Verde MR, Peregalli V, Rodrigo C. Effects of short-term 28% and 100% oxygen on Paco2 and peak expiratory flow rate in acute asthma: a randomised trial. Chest 2003; 124: 1312-1317.
- 7. Wijesinghe M, Perrin K, Healy B, et al. Randomized controlled trial of high concentration oxygen in suspected community-acquired pneumonia. J R Soc Med 2011; 105: 208-216.
- 8. Wijesinghe M, Williams M, Perrin K, et al. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest 2011; 139: 1018-1024.
- 9. Barrera F, Hillyer P, Ascanio G, Bechtel J. The distribution of ventilation, diffusion, and blood flow in obese patients with normal and abnormal blood gases. Am Rev Respir Dis 1973; 108: 819-830.
- 10. Said SI, Banerjee CM. Venous admixture to the pulmonary circulation in human subjects breathing 100 per cent oxygen. J Clin Invest 1963; 42: 507-515.
- 11. Calzavara G, Lusiani GB, Gambari PF, et al. Effetti dell’inalazione di O2 sulla funzione respiratoria dell’obeso. G Clin Med 1969; 50: 310-322.
- 12. Hollier CA, Harmer AR, Maxwell LJ, et al. Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomised crossover study. Thorax 2014; 69: 346-353.
- 13. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017; 72: ii1-ii90.
- 14. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: “Swimming between the flags”. Respirology 2015; 20: 1182-1191.
- 15. Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: Prevalence, effects, and outcome. Am J Med 2004; 116: 1-7.
- 16. Fingleton J, McKinstry S, Pilcher J, et al. Accuracy of change in carbon dioxide measurement for change over time. Respirology 2017; 22: 98.
- 17. Senn O, Clarenbach CF, Kaplan V, et al. Monitoring carbon dioxide tension and arterial oxygen saturation by a single earlobe sensor in patients with critical illness or sleep apnoea. Chest 2005; 128: 1291-1296.
- 18. Kocher S, Rohling R, Tschupp A. Performance of a digital PCO2/SPO2 ear sensor. J Clin Monit 2004; 18: 75-79.
- 19. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319-338.
- 20. Denniston AK, O’Brien C, Stableforth D. The use of oxygen in acute exacerbations of chronic obstructive pulmonary disease: a prospective audit of pre-hospital and hospital emergency management. Clin Med 2002; 2: 449-451.
- 21. Pilcher J, Cameron L, Braithwaite I, et al. Comparative audit of oxygen use in the prehospital setting, in acute COPD exacerbation, over 5 years. Emerg Med J 2013; 32: 234-238.
- 22. Cameron L, Pilcher J, Weatherall M, et al. The risk of serious adverse outcomes associated with hypoxaemia and hyperoxaemia in acute exacerbations of COPD. Postgrad Med J 2012; 88: 684-689.
- 23. Wagstaff TAJ, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia 2007; 62: 492-503.
- 24. Jeffrey AA, Warren PM, Flenley DC. Acute hypercapnic respiratory failure in patients with chronic obstructive lung disease: risk factors and use of guidelines for management. Thorax 1992; 47: 34-40.
- 25. Ambrosino N, Foglio K, Rubini F, et al. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive airways disease: correlates for success. Thorax 1995; 50: 755-757.
- 26. Zavorsky GA, Cao J, Mayo NE, et al. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol 2007; 155: 268-279.
- 27. Rudolf M, Turner JM, Harrison BD, et al. Changes in arterial blood gases during and after a period of oxygen breathing in patients with chronic hypercapnic respiratory failure and in patients with asthma. Clin Sci (Lond) 1979; 57: 389-396.





Abstract
Objective: To compare the effects on transcutaneous carbon dioxide tension (Ptco2) of high concentration and titrated oxygen therapy in medical inpatients with morbid obesity who were not selected for a pre-existing diagnosis of obesity hypoventilation syndrome.
Design: A randomised, crossover trial undertaken between February and September 2015.
Setting: Internal medicine service, Wellington Regional Hospital, New Zealand.
Participants: 22 adult inpatients, aged 16 years or more, with a body mass index exceeding 40 kg/m2.
Interventions: Participants received in random order two 60-minute interventions, with a minimum 30-minute washout period between treatments: titrated oxygen therapy (oxygen delivered, if required, via nasal prongs to achieve peripheral oxygen saturation [Spo2] of 88–92%), and high concentration oxygen therapy (delivered via Hudson mask at 8 L/min, without regard to Spo2). Ptco2 and Spo2 were recorded at 10-minute intervals.
Main outcome measure: Ptco2 at 60 minutes, adjusted for baseline.
Results: Baseline Ptco2 was 45 mmHg or lower for 16 participants with full data (73%). The mean difference in Ptco2 between high concentration and titrated oxygen therapy at 60 minutes was 3.2 mmHg (95% CI, 1.3–5.2 mmHg; P = 0.002).
Conclusion: High concentration oxygen therapy increases Ptco2 in morbidly obese patients. Our findings support guidelines that advocate oxygen therapy, if required in patients with morbid obesity, be titrated to achieve a target Spo2 of 88–92%.
Clinical trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12610000522011.