Summary
- Coal workers’ pneumoconiosis (CWP) is an untreatable but preventable lung disease arising from chronic inhalation of coal dust.
- Recent reports of CWP in Queensland, along with international data, suggest that there is a resurgence in pneumoconiosis.
- The prevalence of CWP varies considerably between countries. In Australia, there is no mandatory reporting system and no national data on the prevalence of CWP.
- The symptoms and manifestations of CWP vary depending on the composition of the inhaled dust, duration of exposure, stage of disease and host-related factors. CWP may develop into progressive massive fibrosis (PMF), which can be fatal.
- Radiological assessment should be performed according to evidence-based standards using the ILO (International Labour Office) International Classification of Radiographs of Pneumoconioses.
- As preventing exposure to coal dust prevents CWP, it is important to implement and enforce appropriate standards limiting exposure. In Australia, these standards currently vary between states and are not in keeping with international understanding of the levels of coal dust that cause disease.
- Longitudinal screening programs are crucial for monitoring the health of coal workers to identify individuals with early-stage disease and prevent progression from mild disease to PMF.
- We recommend:
- standardisation of coal dust exposure limits, with harmonisation to international regulations;
- implementation of a national screening program for at-risk workers, with use of standardised questionnaires, imaging and lung function testing;
- development of appropriate training materials to assist general practitioners in identifying pneumoconiosis; and
- a system of mandatory reporting of CWP to a centralised occupational lung disease register.
“Pneumoconiosis” refers to a group of fibrotic lung diseases caused by the retention of dust in the lung. Coal workers’ pneumoconiosis (CWP), also known as “black lung”, is an irreversible interstitial lung disease resulting from chronic inhalation of coal dust.1 CWP has a long history, with the first case being reported in 1831.2 Workers exposed to coal dust are at risk of a range of chronic lung diseases including CWP,1 silicosis,1 mixed dust pneumoconiosis,3 chronic obstructive pulmonary disease4 and chronic bronchitis.4 In cases of heavy dust exposure, CWP may develop into progressive massive fibrosis (PMF),5 which can be fatal. In 2013, CWP resulted in 25 000 deaths globally.6 Most cases of CWP occur in the setting of poor occupational hygiene and dust control.7
Recent reports of CWP in Australia — six confirmed cases were reported by nominated medical advisers in the Queensland coal industry between May 2015 and February 20168 — are highly concerning and point to a potential decline in exposure control in Australian mines or a failure of the screening process, or both. If true, this is particularly disappointing given the historical success of Australian systems, such as the Joint Coal Board (formed in 19469), in reducing the burden of lung disease in the coal industry through comprehensive screening programs and oversight of dust exposure control. Given the potential resurgence of CWP, it is important that we understand the potential determinants of disease prevention. Here, we consider CWP in the Australian context but do not discuss the other lung diseases attributable to coal dust exposure.
Epidemiology of coal workers’ pneumoconiosis
There is a strong relationship between inhaled dust dose and the risk of developing CWP.10 Airborne respirable dust in coal mines consists of a number of dusts that are potentially dangerous to the lung. It originates from within the coal seam or from adjacent fractured rock and is caused by coal cutting and other operations such as roof bolting. The proportion of different dusts affects the type and severity of lung disease that may develop.
In 1990 and 2013, 29 000 and 25 000 deaths, respectively, were attributed to CWP globally, compared with 55 000 and 46 000 for silicosis.6 In the United States, there has been a resurgence of CWP. While there was a decrease in the prevalence of CWP in coal miners in the US from the 1970s to 1990s (from 6.5% to 2.1%), prevalence then increased from the 1990s to the 2000s (to 3.2%).11 This was accompanied by a substantial increase in the prevalence of coal mine workers with PMF (1990s, 0.14%; 2000s, 0.31%).11 While improvements in the quality of x-rays and reader technique may explain the some of the increase in CWP, the concomitant increase in PMF suggests that this is a true increase in disease prevalence. In the 2010 explosion that killed 29 miners in West Virginia, post mortem examination showed pathological changes consistent with CWP in 17 of 24 victims; 16 of these workers had started working after modern dust limits were applied.12
The recent increase in CWP is concerning and has been attributed to several potential causes: changes in the physicochemical characteristics of the dust, reduction in dust suppression activities, and increased workload (ie, increased exposure).11 CWP is still an important occupational lung disease, even in developed countries, and any reduction in vigilance regarding inhaled coal dust is likely to result in a significant increase in morbidity and mortality.
The prevalence of CWP varies considerably by country. In the United Kingdom, the incidence of CWP declined dramatically between 2004 and 2008 and has remained relatively stable since; it is unclear how these data reflect CWP prevalence in coal workers.13 In some countries, the prevalence of CWP in coal workers remains high, such as China (6.02%) and India (3.03%).14,15 In Australia, there are very few data regarding the true prevalence of CWP in coal workers, although long-term data from Coal Services (which replaced the Joint Coal Board) suggest that there have been no new cases of CWP in New South Wales since the 1980s.16 It is difficult to access information about cases due to a lack of mandatory reporting in Australia.
One retrospective study of pneumoconiosis mortality in Australia found that, of the more than 1000 deaths attributed to pneumoconiosis between 1979 and 2002, only 6% were classified as CWP, with the number of fatalities decreasing steadily over time.17 Data from the National Occupational Health and Safety Commission, based on potentially unreliable workers compensation statistics, suggest that between 2001 and 2003 there were 750 new cases of pneumoconiosis (including CWP, asbestosis and silicosis), with 92 deaths in 2003.18 Mortality from pneumoconiosis in this period of < 1 per 100 000 population is in stark contrast to the 1950s rate of 3.9 per 100 000.18 Again, it is difficult to determine the current prevalence of CWP among coal workers based on these data, and it is likely that CWP incidence is underestimated. One study comparing the prevalence of CWP in the US and NSW suggested that CWP was almost absent in Australian coal workers (prevalence < 0.5%).19 This is despite documented higher levels of dust in NSW19 than in the US, and clear evidence of increases in CWP-related morbidity from international studies, as discussed above. This also contradicts estimates of PMF in Australian coal workers (based on international data), which range from 1.3% to 2.9%.20 These discrepancies point to a need for standardising diagnosis and reporting of CWP nationally, and independent of industry, so that the true burden of disease in coal workers can be accurately monitored.
Symptoms and manifestations of coal workers’ pneumoconiosis
The symptoms and manifestations of CWP vary according to the stage of the disease and the physicochemical properties of the dust that has been inhaled. CWP has a long latency period (usually ≥ 10 years), and individuals with mild disease usually have no symptoms, making early diagnosis difficult at a time when prevention is most effective.1 Symptoms begin with mild cough, followed by increasing breathlessness, wheeze and cough productive of black sputum (melanoptysis) in later stages, accompanied by significant airflow obstruction,21 gas trapping and impaired diffusion capacity.22 Restrictive deficits may also occur as a result of fibrosis,3 and late complications include pulmonary hypertension,23 cor pulmonale24 and death. The symptoms of CWP are non-specific and identical to those of lung disease from other causes. Chronic bronchitis and emphysema are also well documented to occur with coal dust exposure,25 further complicating diagnosis.
Pure carbon, the main constituent of coal, is largely inert. However, the physicochemical properties of processed coal are complex and include organic and inorganic contaminants with known pro-inflammatory and carcinogenic properties, such as silica, iron, cadmium, lead, kaolin, pyrite and polycyclic aromatic hydrocarbons.1,26 The nature and extent of these, along with the physical properties of the carbon particles, have a significant impact on the risk of developing pneumoconiosis.27 Crystalline free silica, the commonest contaminant, independently causes silicosis28 and is often found in high quantities in dust associated with coal mining.29 Thus, CWP and silicosis have significant overlap.
CWP results from the aberrant repair processes that occur when prolonged exposure leads to the activation of pro-inflammatory and pro-fibrotic pathways in the lung.30 Coal dust stimulates pathways that lead to fibrosis due to the cytotoxic effects of the particles31 and the release of pro-inflammatory and pro-fibrotic mediators by cells responding to the particles.30 Central to this is the capacity of coal particles to produce abundant reactive oxygen species32 and induce oxidative stress.33
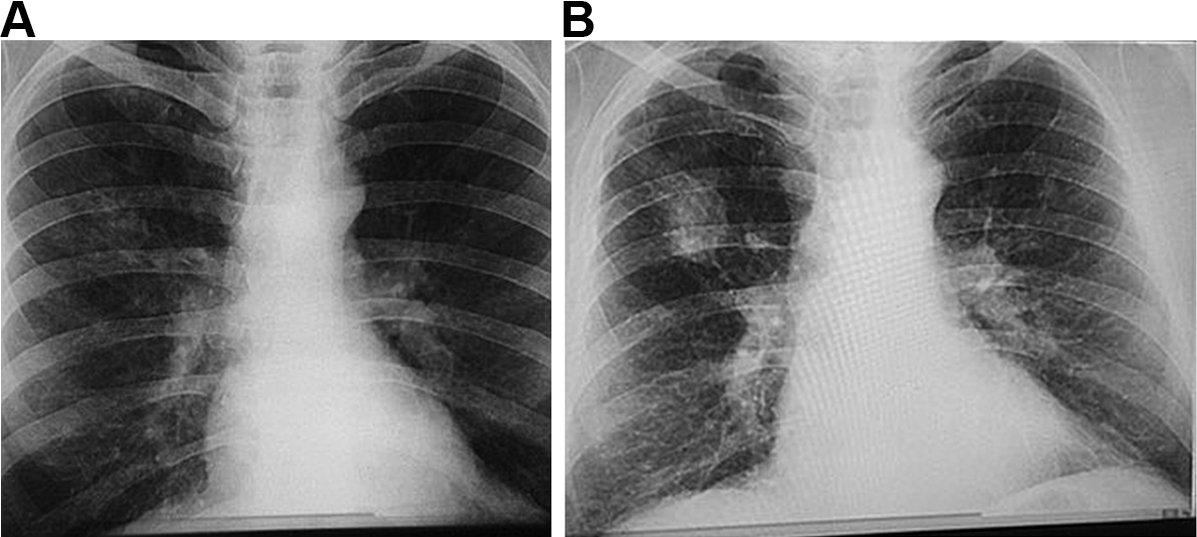
The manifestations of CWP can vary greatly between individuals, depending on the composition of the dust and duration of exposure, as well as host-related factors.34 Accumulation of dust occurs initially in the walls of the respiratory bronchioles, the adventitia of the blood vessels and the bronchoalveolar canals. Collections of dust-laden macrophages accumulate in the walls of the airways, particularly at their bifurcations, and in adjacent alveoli.1 Fibrous tissue is deposited, which later shrinks and leads to distortion of local lung structures.34 With increasing release of inflammatory mediators and deposition of fibrous tissue, these “macules” become larger and develop into more organised, dense, central, dust-pigmented lesions called micronodules,1 which can be palpated in the lung and seen on a chest x-ray (Box 1). Many larger rounded nodules then develop, particularly in the mid and upper zones of the lungs. Subsequently, centriacinar emphysema develops.35
Eventually, large masses of coal dust (Box 2), lymphocytes, dust-laden macrophages, reticulin and collagen may converge to form areas of PMF. These usually occur in the upper posterior parts of the lungs and appear as large rounded masses on the chest x-ray34 (Box 1, B). The presence of PMF represents “complicated” CWP and is associated with increasing symptoms and mortality.34
Radiological assessment relies on systematic objective assessment of good-quality chest x-rays. Scoring is important and should be performed to strict standards using the ILO (International Labour Office) International Classification of Radiographs of Pneumoconioses.36 The ILO classification provides a means of systematically recording the chest radiographic abnormalities that occur in any type of pneumoconiosis. The classification does not imply legal definitions of pneumoconioses for compensation purposes.36 While low-dose, high-resolution computed tomography (HRCT) scans are more sensitive for early disease,37 CT scanning is not currently used for screening purposes.
“Simple” pneumoconiosis is characterised by small, ill defined, rounded opacities in the outer thirds of the lung fields and the mid and upper zones. These can be categorised by size (categories q, r and s) and by density (categories 1–3). Identifiable radiological changes often occur well before changes in lung function or clinically significant disease. The whole of the lung fields may be involved in stage 3. PMF appears as rounded, sausage-shaped or ovoid opacities greater than 1 cm in diameter, which are well demarcated from the adjacent lung and may vanish if the contents are expectorated. Standard x-rays are available from the ILO for comparison purposes. Documentation of results includes an assessment of the technical quality of the chest x-ray, as well as relevant findings, and each detailed finding is assigned a standard code that facilitates documentation, diagnosis and monitoring.36
Prevention of coal workers’ pneumoconiosis
As the only cause of CWP is coal dust, prevention is straightforward — preventing exposure to coal dust prevents disease. This is important, as no effective treatments for CWP exist. Like all occupational diseases, prevention requires adherence to appropriate environmental standards and occupational health and safety guidelines by employers and workers.
Standards exist for limiting exposure to the respirable fraction of coal dust in most industrial settings. However, these standards vary considerably between states in Australia. For example, in Queensland the standard is 3.0 mg/m3, while in NSW it is 2.5 mg/m3.19 Both of these are significantly less stringent than the current US standard of 1.5 mg/m3.19 The Australian Institute of Occupational Hygienists has recommended that the limit be reduced to 1.0 mg/m3,38 and it could be argued that it should be even lower. The discrepancies in limits are compounded by variation in testing protocols between regions. For example, in Queensland, monitoring of dust exposure includes the travel time between the mine entrance and the coal face, whereas in NSW, exposure is only monitored during the individual miner’s period of underground work.19
Knowing the legislated standard for coal dust levels, it is up to the employer to implement measures to prevent exposure. However, the extent of implementation may depend on the costs associated with dust mitigation, which may explain why smaller mines tend to have a higher incidence of CWP.11 The management and oversight of dust sampling and monitoring is an important factor that can have a big impact on dust exposure; the intricacies of this are beyond the scope of this review. Given the variation in standards between states, in both dust monitoring and suppression, it is not possible to assess the overall extent of compliance with standards or the implementation of dust suppression strategies throughout Australia. However, a recent report by the Queensland Mines Inspectorate has raised concerns about the level of exposure in some situations,39 whereby 60% of mines exposed longwall operators to levels equal to or greater than the exposure limit in 2014, compared with 10% in 2012. Similarly, there have been increases in the percentage of mines that have exceeded regulatory limits (0 in 2012 v 25% in 2014).39
Taken together, these observations indicate that Australian standards are not based on the international understanding of the levels of coal dust that are likely to cause disease, and there are no consistent standards for monitoring dust levels. There is also some evidence to suggest that regulatory compliance may be a problem.
Screening for coal workers’ pneumoconiosis
Screening programs are crucial for monitoring the health of coal workers to identify individuals with early-stage disease and prevent progression from mild asymptomatic disease to PMF. Screening is mandatory in other countries and has been very effective at reducing the prevalence of pneumoconiosis in workers at risk of exposure to occupational dust.40 In situations where screening is voluntary, there is a low (about 40%) participation rate.19 The World Health Organization recommends that all workers exposed to coal and silica dust undergo lifelong health surveillance, including a baseline assessment (with chest x-ray) before commencing work, annual spirometry and symptom questionnaires, and follow-up chest x-rays every 2–5 years.41 Surveillance should continue after exposure and records should be kept for 30 years or longer after cessation of employment. Health surveillance is usually financed by the employer.
X-ray assessment using the ILO method is the current international standard for identifying disease.36 Questionnaires and spirometry are also effective in detecting other chronic lung diseases associated with dust exposure and can prompt referral to a respiratory specialist. Lung function assessment will identify respiratory disorders not visible on imaging and allow tracking of individual trajectories of lung decline. A computerised program (Spirometry Longitudinal Data Analysis [SPIROLA] software) is available free from the US National Institute for Occupational Safety and Health (NIOSH) to identify workers whose decline in lung function is greater than normal.
In the US, the NIOSH requires radiologists to be certified as “B readers” for classifying pneumoconiosis to ILO standards.42 This is not a requirement in Europe or Australia. The B Reader Program is a proficiency program to provide a pool of qualified readers capable of accurately using the ILO system,42 which has recently incorporated modern digital technologies.34 Use of digital techniques may improve the reproducibility of small-opacity profusion classification in some respects, but could also slightly reduce the frequency with which some readers identify large opacities.34 HRCT is acknowledged as being more sensitive than chest radiography for detecting parenchymal and pleural abnormalities and interstitial fibrosis,37 but it is limited by equipment availability, costs and radiation exposure, although modern CT scanners use a much lower radiation dose that is comparable to old chest x-rays.43 In Australia, the proficiency of radiologists for reporting surveillance films in accordance with ILO methods is determined by the Royal Australian and New Zealand College of Radiologists (RANZCR).
Data from a screening program must be carefully maintained to allow longitudinal assessment, which may identify important changes over time. A comprehensive surveillance program also requires a process for referral to specialist respiratory physicians and a means of explaining results to the worker, preferably with a written record of examination results.
Conclusions
Recent reports of CWP in Australia and international epidemiological data suggest an increase in CWP prevalence among coal workers and are a major concern. Given that CWP is a preventable but untreatable disease, no new cases should occur. Disease eradication must be the aim. We suggest the following actions, which are summarised in Box 3.
1. Dust exposure limits and monitoring
The current Australian standards for coal dust exposure limits, which vary between states, are less stringent than international recommendations, and exposure monitoring protocols vary considerably between sites. We strongly urge that the Australian guidelines be reviewed on the basis of current knowledge of CWP, in line with international standards, and that exposure limits and monitoring protocols are nationally standardised according to best practice guidelines.
2. Screening
We strongly advocate for a comprehensive screening program for workers at risk of exposure to coal or silica dust using a protocol based on international guidelines, which includes a questionnaire, medical imaging and lung function testing including measurement of diffusion capacity. This should be funded by the employer but preferably evaluated by physicians and radiologists not employed by the coal companies. We recommend implementing recent advances in lung imaging, including digital radiography and storing data in a single de-identified central system, which could be accessible to workers, unions, government agencies and employers. Workers should be given a copy of their results after each screening. The WHO recommends data storage for at least 30 years after the worker’s retirement, and we endorse this recommendation.
We acknowledge that chest radiography can be insensitive and non-specific for the diagnosis of CWP, but screening with low-dose CT is currently impractical for reasons of cost, availability and convenience. Digital radiography using ILO standards should be performed at the commencement of coal dust exposure and at least every 3 years, or more frequently, depending on the results of ongoing assessment. Sending radiological images overseas is unnecessary and the RANZCR register of radiologists who can assess to ILO standards should be used. We believe that further research is needed to examine the utility of more modern methods of early diagnosis; namely, the use of low-dose HRCT screening.
Workers who show an accelerated decline in lung function or a change in radiological or questionnaire scores should be recommended for further investigation with HRCT and full lung function testing, including measurement of gas transfer performed by accredited laboratory personnel according to international standards and interpreted by a specialist respiratory physician. Workers should be referred early to specialist respiratory physicians with an interest in occupational lung disease, and the costs of such investigations should be borne by employers. Where pneumoconiosis is diagnosed, we recommend a system of mandatory reporting to a centralised occupational lung disease register.
3. Medical practitioner training and referral
Pneumoconiosis is rarely diagnosed in the general practice setting. We suggest that training materials for medical practitioners be developed to assist with identifying current or retired workers at risk of pneumoconiosis. We also suggest that all dust-exposed workers with significant respiratory symptoms, whether in an existing surveillance program or after retirement, be referred to a specialist respiratory physician, preferably with expertise in occupational lung disease.
4. A centralised occupational lung disease register
Current data regarding occupational lung disorders in Australia are inadequate. The results of surveillance for CWP should be made publicly available and stored in a central repository. Thus, individual changes in respiratory health could be monitored, as well as changes in prevalence and incidence, and the system could be used to detect potential difficulties in surveillance and prevention processes. CWP and other occupational lung diseases should be made notifiable diseases, so that all diagnosed cases are recorded. Such information should be used to close the loop and feed back to employers to allow early implementation of change.
It is unacceptable that any new cases of CWP should be occurring in Australia in 2016, and our aim should be to eliminate CWP in Australia altogether.
Box 1 – Chest x-rays of a coal worker (A) showing background nodulation and early progressive massive fibrosis (PMF) in the right upper zone, and (B) 12 years later, showing PMF
Box 2 – Gough section of a coal worker’s lung showing coal workers’ pneumoconiosis with progressive massive fibrosis

Box 3 – Recommendations for control of coal workers’ pneumoconiosis (CWP) endorsed by the Thoracic Society of Australia and New Zealand
Goal: Eliminate CWP in Australia
- 1. Exposure limits and monitoring protocols
- Standardise across Australia and harmonise to international recommendations
- 2. Screening
- Develop and implement a national screening program for at-risk workers
- Questionnaire, imaging, lung function testing
- 3. Medical workforce training
- 4. A centralised occupational lung disease register
Provenance: Not commissioned; externally peer reviewed.
- Graeme R Zosky1
- Ryan F Hoy2
- Elizabeth J Silverstone3
- Fraser J Brims4,5
- Susan Miles6,7
- Anthony R Johnson8
- Peter G Gibson6
- Deborah H Yates9
- 1 School of Medicine, University of Tasmania, Hobart, TAS
- 2 Allergy, Immunology and Respiratory Medicine, Alfred Hospital, Melbourne, VIC
- 3 Department of Medical Imaging, St Vincent's Hospital, Sydney, NSW
- 4 Department of Respiratory Medicine, Sir Charles Gairdner Hospital, Perth, WA
- 5 School of Population Health, University of Western Australia, Perth, WA
- 6 Department of Respiratory and Sleep Medicine, John Hunter Hospital, Newcastle, NSW
- 7 Faculty of Medicine and Public Health, University of Newcastle, Newcastle, NSW
- 8 Thoracic Medicine, Liverpool Hospital, Sydney, NSW
- 9 Department of Thoracic Medicine, St Vincent's Hospital, Sydney, NSW
Peter Gibson is a member of the MJA Editorial Advisory Committee.
- 1. Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect 2000; 108 Suppl 4: 675-684.
- 2. Gregory JC. Case of particular black infiltration of the whole lung resembling melanosis. Edinburgh Med Surg J 1831; 36: 389-394.
- 3. Petsonk EL, Rose C, Cohen R. Coal mine dust lung disease: new lessons from old exposure. Am J Respir Crit Care Med 2013; 187: 1178-1185.
- 4. Wouters EF, Jorna TH, Westenend M. Respiratory effects of coal dust exposure: clinical effects and diagnosis. Exp Lung Res 1994; 20: 385-394.
- 5. Shennan DH, Washington JS, Thomas DJ, et al. Factors predisposing to the development of progressive massive fibrosis in coal miners. Br J Ind Med 1981; 38: 321-326.
- 6. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117-171.
- 7. Vallyathan V, Landsittel DP, Petsonk EL, et al. The influence of dust standards on the prevalence and severity of coal worker’s pneumoconiosis at autopsy in the United States of America. Arch Pathol Lab Med 2011; 135: 1550-1556.
- 8. The Senate Select Committee on Health. Fifth interim report. Black Lung: “It has buggered my life”. April 2016. Canberra: Senate Printing Unit, 2016. http://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Health/Health/Fifth_Interim_Report (accessed Apr 2016).
- 9. Niall P, Wiles AN. Special Medical Boards for the NSW coal industry: an historical overview. J Occup Health Saf Aust New Zealand 2002; 18: 431-435.
- 10. Liu H, Tang Z, Yang Y, et al. Identification and classification of high risk groups for coal workers’ pneumoconiosis using an artificial neural network based on occupational histories: a retrospective cohort study. BMC Public Health 2009; 9: 366.
- 11. Laney AS, Attfield MD. Coal workers’ pneumoconiosis and progressive massive fibrosis are increasingly more prevalent among workers in small underground coal mines in the United States. Occup Environ Med 2010; 67: 428-431.
- 12. McAteer JD, Beall K, Beck JA Jr, et al. Upper Big Branch. The April 5, 2010, explosion: a failure of basic coal mine safety practices. Charleston, WV: National Technology Transfer Center, 2011. http://www.nttc.edu/programs&projects/minesafety/disasterinvestigations/upperbigbranch/UpperBigBranchReport.pdf (accessed Apr 2016).
- 13. UK Health and Safety Executive. Pneumoconiosis. Merseyside: HSE, 2015. http://www.hse.gov.uk/statistics/causdis/pneumoconiosis (accessed Apr 2016).
- 14. Mo J, Wang L, Au W, Su M. Prevalence of coal workers’ pneumoconiosis in China: a systematic analysis of 2001-2011 studies. Int J Hyg Environ Health 2014; 217: 46-51.
- 15. Parihar YS, Patnaik JP, Nema BK, et al. Coal workers’ pneumoconiosis: a study of prevalence in coal mines of eastern Madhya Pradesh and Orissa states of India. Ind Health 1997; 35: 467-473.
- 16. Coal Services. Coal Services annual report 2014-2015. Sydney: Coal Services, 2015. http://www.coalservices.com.au/annualreports.aspx (accessed Apr 2016).
- 17. Smith DR, Leggat PA. 24 years of pneumoconiosis mortality surveillance in Australia. J Occup Health 2006; 48: 309-313.
- 18. Australian Institute of Health and Welfare. Chronic respiratory diseases in Australia: their prevalence, consequences and prevention (AIHW Cat. No. PHE 63). Canberra: AIHW, 2005. http://www.aihw.gov.au/publication-detail/?id=6442467751 (accessed Apr 2016).
- 19. Joy GJ, Colinet JF, Landen DD. Coal workers’ pneumoconiosis prevalence disparity between Australia and the United States. Atlanta: National Institute for Occupational Safety and Health, 2012. http://www.cdc.gov/niosh/mining/UserFiles/Works/pdfs/cwppd.pdf (accessed Apr 2016).
- 20. Australian Safety and Compensation Council. Occupational respiratory diseases in Australia, April 2006. Canberra: ASCC, 2005. http://www.safeworkaustralia.gov.au/sites/SWA/about/Publications/Documents/114/OccupationalrespiratoryDiseases_Australia_2006_ArchivePDF.pdf (accessed Apr 2016).
- 21. Jorna TH, Schins RP, Lenaerts L, et al. Airflow obstruction and monocyte TNF release in coal workers. Exp Lung Res 1994; 20: 421-431.
- 22. Wang X, Yu IT, Wong TW, Yano E. Respiratory symptoms and pulmonary function in coal miners: looking into the effects of simple pneumoconiosis. Am J Ind Med 1999; 35: 124-131.
- 23. Akkoca Yildiz O, Eris Gulbay B, Saryal S, Karabiyikoglu G. Evaluation of the relationship between radiological abnormalities and both pulmonary function and pulmonary hypertension in coal workers’ pneumoconiosis. Respirology 2007; 12: 420-426.
- 24. Lapp NL, Parker JE. Coal workers’ pneumoconiosis. Clin Chest Med 1992; 13: 243-252.
- 25. Coggon D, Newman Taylor A. Coal mining and chronic obstructive pulmonary disease: a review of the evidence. Thorax 1998; 53: 398-407.
- 26. Achten C, Hofmann T. Native polycyclic aromatic hydrocarbons (PAH) in coals — a hardly recognized source of environmental contamination. Sci Total Environ 2009; 407: 2461-2473.
- 27. Huang X, Li W, Attfield MD, et al. Mapping and prediction of coal workers’ pneumoconiosis with bioavailable iron content in the bituminous coals. Environ Health Perspect 2005; 113: 964-968.
- 28. Leung CC, Yu ITS, Chen W. Silicosis. Lancet 2012; 379: 2008-2018.
- 29. Cohen RAC, Patel A, Green FH. Lung disease caused by exposure to coal mine and silica dust. Semin Respir Crit Care Med 2008; 29: 651-661.
- 30. Vanhee D, Gosset P, Wallaert B, et al. Mechanisms of fibrosis in coal workers’ pneumoconiosis. Increased production of platelet-derived growth factor, insulin-like growth factor type I, and transforming growth factor beta and relationship to disease severity. Am J Respir Crit Care Med 1994; 150: 1049-1055.
- 31. Vallyathan V. Generation of oxygen radicals by minerals and its correlation to cytotoxicity. Environ Health Perspect 1994; 102 Suppl 10: 111-115.
- 32. Pinho RA, Silveira PC, Silva LA, et al. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res 2005; 99: 355-360.
- 33. Pinho RA, Bonatto F, Andrades M, et al. Lung oxidative response after acute coal dust exposure. Environ Res 2004; 96: 290-297.
- 34. Parkes WR. Occupational lung disorders. 3rd ed. Oxford: Butterworth-Heinemann, 1994.
- 35. Ryder R, Lyons JP, Campbell H, Gough J. Emphysema in coal workers’ pneumoconiosis. Br Med J 1970; 3: 481-487.
- 36. International Labour Organization. Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses. Revised edition 2011. Geneva: ILO, 2011. http://www.ilo.org/safework/info/publications/WCMS_168260/lang--en/index.htm (accessed Apr 2016).
- 37. Meijer E, Tjoe Nij E, Kraus T, et al. Pneumoconiosis and emphysema in construction workers: results of HRCT and lung function findings. Occup Environ Med 2011; 68: 542-546.
- 38. Australian Institute of Occupational Hygienists Exposure Standards Committee. Dusts not otherwise specified (dust NOS) and occupational health issues: position paper. Melbourne: AIOH, 2014. http://www.aioh.org.au/documents/item/16 (accessed Apr 2016).
- 39. Harrison P. Queensland Mines Inspectorate annual performance report 2014–15. Queensland: Queensland Department of Natural Resources and Mines, 2015. https://www.dnrm.qld.gov.au/__data/assets/pdf_file/0008/311498/qld-mines-inspectorate-annual-performance-report-2014-15.pdf (accessed Apr 2016).
- 40. Scarisbrick DA, Quinlan RM. Health surveillance for coal workers’ pneumoconiosis in the United Kingdom 1998–2000. Ann Occup Hyg 2002; 46 Suppl 1: 254-256.
- 41. Wagner GR. Screening and surveillance of workers exposed to mineral dust. Geneva: World Health Organization, 1996. http://www.who.int/occupational_health/publications/oehmineraldust.pdf (accessed Apr 2016).
- 42. National Institute for Occupational Safety and Health. Chest radiography. The NIOSH B Reader Program. Atlanta: NIOSH, 2012. http://www.cdc.gov/niosh/topics/chestradiography/breader.html (accessed Apr 2016).
- 43. Singh S, Kalra MK, Ali Khawaja RD, et al. Radiation dose optimization and thoracic computed tomography. Radiol Clin North Am 2014; 52: 1-15.





Arthur (Bill) WilliamMusk
While coal workers’ pneumoconiosis has not been an issue in Western Australia due to the nature of the coal deposits in this state, there has been a long history of pneumoconioses resulting from silica and asbestos exposure with an epidemic of silicosis in the 1920s, 30s and 40s and of asbestosis in the 1950s. In earlier years the occurrence of tuberculosis clouded the issues but prompted the imposition of a system for disease surveillance in the mining industry in 1926 such that annual chest X-rays were required on all mine workers. While the incidence and prevalence of silicosis were measured and X-rays read in a standard way (analogous to the ILO Classification) the relation between dust measurements and disease incidence was not effected until the 1950s. Dr James McNulty, past State Mines’ Medical Officer and also past Health Commissioner in WA insisted that dust control in mines was a medical issue requiring an engineering solution in order eliminate silicosis. More accurate dust measurement with medical input and correlation with chest X-rays were undertaken.
As a result of knowledge of the incidence of dust disease limitations were placed on dust exposure in mines but silicosis incidence only declined when working conditions improved and diesel equipment and better ventilation were introduced. By the 1970s silicosis was virtually eradicated.
The routine surveillance chest X-rays for miners introduced in WA in 1926 and continued by the Perth Chest Clinic was disbanded in the 1980s in the belief that mining conditions were safe and silicosis had been completely eliminated. Health surveillance of mine workers is now undertaken by individual mining companies so that, as in Queensland there is now no central repository to allow timely detection of early disease cases that would prompt investigation of working conditions.
So the recommendations of Zosky et al are not new and should be implemented: without acknowledging the history of disease surveillance and control in mine workers they are proposing a system that has already been proven to be successful, at least in Western Australia but also Australia-wide as well as in the United Kingdom, USA and elsewhere!
Ref: Fitzgerald Criena, Turning Men Into Stone. A Social and Medical History of Silicosis in Western Australia. Hesperian Press, Perth, 2016.
AW Musk
CJ Fitzgerald
NH deKlerk
Competing Interests: No relevant disclosures
Prof Arthur (Bill) WilliamMusk
Sir Charles Gairdner Hospital