Clinical record
A 61-year-old American aid worker was transferred to Royal Darwin Hospital from Timor-Leste with fever and rash. He had worked in Timor for 1 year and was in good health apart from an episode of falciparum malaria treated 9 months previously. He described headache, myalgia and fatigue for 7 days, and 6 days of fever and chills. On Day 2 of illness, he attended a Timorese clinic where an unidentified blood test was reported positive for falciparum malaria. Despite initial treatment with sulfadoxine–pyrimethamine and 3 days of atovaquone–proguanil, his fever and chills persisted. After a further positive test result for falciparum malaria at the same laboratory, he attended a referral clinic on the same day, where thick film blood examination and a histidine-rich protein 2 antigen test were negative for malaria. He was noted to have an erythematous truncal rash and switched to artemether–lumefantrine. Because of ongoing fever, he was evacuated to Australia with provisional diagnoses of malaria or, given the rash, dengue fever.
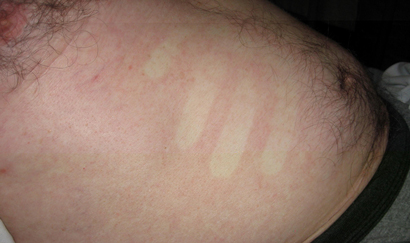
On arrival at our hospital, the patient had a temperature of 38.3°C; oxygen saturation of 94%; a confluent, macular, blanching, non-pruritic rash on his trunk (Figure); and a fine, petechial rash on his ankles. He had bibasal inspiratory crackles but no peripheral oedema. A chest x-ray showed small bilateral pleural effusions. Admission haematology showed anaemia (haemoglobin level, 131 g/L [reference interval (RI), 135–180 g/L]); thrombocytopenia (platelet count, 17 × 109/L [RI, 140–400 × 109/L]); normal total white cell count (4.4 × 109/L [RI, 4.0–11.0 × 109/L]); lymphopenia (lymphocyte count, 0.4 × 109/L [RI, 1.0–4.0 × 109/L]); and neutropenia (neutrophil count, 0.4 × 109/L [RI, 2.0–8.0 × 109/L]). Blood films were negative for malaria parasites but showed band forms, toxic granulation and reactive lymphocytes. A repeat histidine-rich protein 2 antigen test for Plasmodium falciparum returned a negative result. His alanine aminotransferase level (67 IU/L; RI, < 34 IU/L), creatinine concentration (142 μmol/L; RI, 46–99 μmol/L) and activated partial thromboplastin time (51 s; RI, 26–41 s) were all elevated.
Further history revealed that his symptoms had commenced on arrival in Bali en route to Timor, after a 4-week holiday on his orchard in Seminole County, Florida, United States. On specific enquiry, he described multiple tick bites over a 1-week period up until 4 days before symptom onset. With this information, the differential diagnosis shifted to tick-borne infections, with Rocky Mountain spotted fever (RMSF) and erhlichiosis considered most likely. Treatment with doxycycline was commenced promptly (200 mg loading dose followed by 100 mg twice daily) and continued for 5 days after defervescence. Within 72 hours, his symptoms improved, with resolution of fever, rash, thrombocytopenia, renal impairment and coagulopathy.
Serological tests for dengue fever virus and Rickettsia rickettsii (RMSF) on paired sera were negative. Dengue NS1 antigen testing and blood cultures were negative. Serology (IgM and IgG) by microimmunofluoresence for Ehrlichia chaffeensis showed a titre increase from 1 : 512 to 1 : 8192 over 8 days. Q fever and rickettsial serological tests were negative. The patient was also tested for Anaplasma phagocytophilum, another tick-borne infection endemic to the US which can present with a similar illness. Paired sera showed an increase in A. phagocytophilum titres from 1 : 256 to 1 : 2048. The lower titre rise compared with E. chaffeensis was considered to most likely represent a cross-reaction rather than dual infection.1
We did not look for intracytoplasmic morulae characteristic of E. chaffeensis infection using buffy coat examination, as it is considered insensitive compared with serology.2 The combination of serological, clinical and laboratory findings supported a diagnosis of human monocytic ehrlichiosis acquired in the US.
Ehrlichia chaffeensis, an obligate intracellular pathogen in the Anaplasmataceae family, is a tick-borne pathogen found predominantly in the southern and eastern states of the US.1 It is not endemic in Australia or Timor-Leste, and ehrlichiosis has not previously been reported in Australia (although Anaplasma platys has been found in Australian dogs). After propagation in monocytes, it causes fever, headache, myalgia, thrombocytopenia and leukopenia, with rash occurring in about 30% of cases.1,2 Complications include shock, meningoencephalitis, coagulopathy, acute kidney injury and cardiac failure.1,2 Reported mortality is about 3%, with fatal outcome linked to age and delayed diagnosis and treatment.1,2 Human monocytic ehrlichiosis shares many clinical features with the other US tick-borne rickettsial and rickettsia-like diseases, RMSF and anaplasmosis, with rash more common in RMSF but rare in anaplasmosis. While these may be difficult to differentiate,2-4 all respond well to doxycycline but not to β-lactam antibiotics.2
This case illustrates the importance of obtaining a complete travel and exposure history when assessing febrile travellers, and not just their most recent travel. A reliance on the most recent area of residence and failure to obtain a history of US tick bites led to initial misdiagnoses of malaria and dengue fever, and delayed the initiation of treatment with potentially life-saving doxycycline. The initial unidentified positive malaria diagnoses in an unaccredited Timorese clinic laboratory were not reproducible and were thought to have been false-positives. False-positive microscopy and overdiagnosis of malaria is common in malaria-endemic areas.5 The timing of symptom onset, acute kidney injury and left shift and toxic granulation on blood films4 were not suggestive of dengue fever (nor the rash or left shift for malaria), and all provided further clues to the correct diagnosis.Lessons from practice
Always obtain a complete travel and exposure history when assessing febrile travellers, and not just their most recent travel.
Reliance on most recent travel in a malaria- and dengue-endemic area led to erroneous diagnoses of malaria and dengue.
Consider the possibility of tick-borne infections in patients presenting with fever and rash.
Early initiation of doxycycline is potentially life-saving in ehrlichial and rickettsial infections with mortality linked to delayed diagnosis and inappropriate treatment.
- 1. Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichiosis in humans: epidemiology, clinical presentation, diagnosis and treatment. Clin Infect Dis 2007; 45 Suppl 1: S45-51.
- 2. Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis--United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 2006; 55 (RR-4): 1-27.
- 3. Chen LF, Sexton DJ. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am 2008; 22: 415-432.
- 4. Hamilton KS, Standaert SM, Kinney MC. Characteristic peripheral blood findings in human ehrlichiosis. Mod Pathol 2004; 17: 512-517.
- 5. Strøm GE1, Haanshuus CG, Moyo S, et al. Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malar J 2013; 12: 228.





We thank Chelsea Nguyen of the Australian Rickettsial Reference Laboratory for performing the ehrlichial serology. Nicholas Anstey is supported by a National Health and Medical Research Council Practitioner Fellowship.
No relevant disclosures.