Clinical record
A previously healthy 18-year-old dark-skinned Indigenous man was incarcerated in a juvenile detention centre in New South Wales for 3.5 years from 2010 to 2013. Juvenile detention limits outdoor activity and, consequently, exposure to sunlight. Young people are confined indoors for schooling and other programs, with additional periods of cell lockdowns to accommodate detainee movements and staff handovers. Periods outdoors involve bursts of strenuous physical activity, mostly team sports or swimming. Further, detention centre policy requires young people to wear T-shirts and hats, and to use sun protection factor 30+ sunscreen when outdoors.
On entering custody, the patient's weight was 65 kg, with a healthy body mass index (BMI) of 21 kg/m2 (reference interval [RI], 18.5–24.9 kg/m2). Full blood count, urea, electrolyte and creatinine levels and liver function test results were normal, and a blood-borne virus screen returned a negative result. He had a strong family history of type 2 diabetes in his mother and maternal grandmother, and, reportedly, of hypercholesterolaemia and early cardiovascular death in his father and paternal grandfather.
Seven months after incarceration, the man developed auditory and visual hallucinations and was noted to be withdrawn and depressed, with long periods spent resting in his cell owing to fatigue. He was commenced on the antipsychotic quetiapine 150 mg at night and the antidepressant fluoxetine 20 mg in the morning. At commencement of these medications, his weight was 89 kg (BMI, 29 kg/m2). Baseline pathology tests were not repeated at this time.
Six months after commencement of psychotropic medications, his weight had increased a further 25 kg to 114 kg and he was morbidly obese (BMI, 36 kg/m2), with phenotypes of metabolic syndrome including central obesity (waist circumference, 108 cm [RI, < 94 cm]), hyperlipidaemia (total cholesterol, 7.8 mmol/L [RI, < 5.5 mmol/L]; low-density lipoprotein cholesterol, 4.9 mmol/L [RI, < 4.0 mmol/L]; high-density lipoprotein cholesterol, 0.8 mmol/L [RI, > 1.0 mmol/L]), elevated triglyceride level (2.28 mmol/L [RI, < 2.0 mmol/L]), and fatty liver disease (γ-glutamyl transferase, 83 U/L [RI, 0–60 U/L]; alkaline phosphatase, 208 U/L [RI, 30–110 U/L]; alanine transaminase, 72 U/L [RI, 0–55 U/L]; aspartate transaminase, 46 U/L [RI, 0–45 U/L]) (Figure). Blood pressure and thyroid-stimulating hormone levels were within normal limits. With concerns about his obesity and metabolic derangements, quetiapine was ceased. He received counselling for dietary restriction (portion control, low saturated fat diet, reduction of energy-dense snacks) and, in particular, was encouraged to avoid the additional bread, butter and sugary drinks that are available to supplement meals. Increased physical activity was encouraged. An off-label trial of metformin was commenced, given the evidence for weight benefits in antipsychotic recipients,1 and increased to 1 g twice daily over the following 4 weeks.
Three months later, the patient's fasting lipid levels remained similarly elevated despite lifestyle changes, and he agreed to trial atorvastatin 10 mg daily. He was also permitted to take recreational leave from the centre and commenced thrice-weekly training with the local football club.
Three weeks after commencing atorvastatin, the patient complained of worsening fatigue but denied having muscle tenderness, myalgia or cramping. Creatine kinase (CK) levels were normal at atorvastatin commencement, but had risen to 350 U/L (RI, < 170 U/L). Atorvastatin dosage was reduced to 5 mg in the morning, metformin was continued and fluoxetine was ceased.
Serial changes in the patient's CK levels are shown in the Figure. Five weeks after atorvastatin commencement, lipid levels had improved but CK levels continued to rise and all medications were ceased. There were concerns regarding rhabdomyolysis, but urinalysis results, estimated glomerular filtration rate and renal function remained normal. His physical symptoms remained unchanged. The patient was encouraged to rest and drink plenty of water. He continued to play competition football. CK levels continued to rise, peaking at 3042 U/L.
Serum 25-hydroxyvitamin D levels were found to be low, and he was treated with cholecalciferol (vitamin D3) 1000 IU daily, increasing temporarily to 4000 IU daily after endocrinologist consultation. Serial CK and 25-hydroxyvitamin D levels showed slow improvement initially, with substantial improvements contemporaneous with aggressive vitamin D supplementation (Figure). The patient continued with lifestyle strategies and (in concert with cessation of psychotropic medications) lost 10 kg in weight, but lipid levels remained elevated. Fluoxetine was recommenced by the treating psychiatrist at 20 months because of concerns regarding the patient's mood.
At the conclusion of his sentence, the patient was released from custody and referred to the local Aboriginal Medical Service for continuing management of his hypercholesterolaemia, myositis, and metabolic and mental health problems.
Indigenous Australians have a reduced life expectancy of up to 20 years compared with non-Indigenous Australians and, by 40 years of age, are 10 times more likely to suffer premature cardiac-related death.2 In recognition of this, the Indigenous Chronic Disease Package (through Closing the Gap initiatives) encourages the use of statins, recommending treatment at lower lipid thresholds.3
There is evidence that Indigenous populations may be at higher risk of statin-related myopathy owing to a higher risk of vitamin D deficiency,4 higher rates of human T-cell lymphotropic virus type 1 infections causing polymyositis5 and, possibly, genetic susceptibility to statin-associated myotoxic effects (the SLCO1B1 gene prevalent in other indigenous populations6). Other risk factors predisposing our patient to statin-related myopathy were his age, strenuous exercise, mild hepatic dysfunction and concomitant use of fluoxetine (a CYP3A4 inhibitor).4,7,8 As CK elevation persisted after atorvastatin cessation, the differential diagnosis was necrotising autoimmune myopathy, previously described in indigenous patients with persistent myopathy.9 The recommencement of fluoxetine at 20 months may also have perpetuated the elevated CK level.
Almost 12 000 people are incarcerated in NSW, with a quarter being Indigenous Australians and at greatest risk of vitamin D deficiency.10 This is the first report of statin-related myopathy in an Indigenous adolescent or an incarcerated person. It is worth noting by other clinicians who work with Indigenous and incarcerated groups that the risk factors for this patient's “perfect storm” were not unusual — metabolic syndrome, vitamin D deficiency, and use of statins in the context of mental illness and concomitant psychotropic medication use.1,2
This report highlights the need for monitoring of vitamin D levels and supplementation (with an argument for easier access to injectable vitamin D in this group), with pre-statin counselling, particularly for those at high risk of statin-related myopathy — Indigenous Australians, youths, females, and those serving lengthy custodial sentences.
This case also highlights the detrimental effects of antipsychotics on weight and metabolic risk. An international declaration supporting young people with psychosis11 has delineated the obligations of health care providers to prevent weight gain and metabolic complications that contribute to the 25-year shortfall in life expectancy in people with severe mental illness.
In addition to these learning points, there are the obvious problems of the unmet health needs and human tragedy in this vulnerable patient group: a baseline high metabolic risk associated with Aboriginality and family medical history, the constraints of incarceration exacerbating the risk of vitamin D deficiency, and a doubling of weight resulting in rapid-onset obesity secondary to antipsychotic use. Co-prescription of lifestyle interventions at the time of commencing antipsychotic therapy is essential. In addition, metformin has proven efficacy in abrogating weight gain following antipsychotic commencement, with its use encouraged in patients who make clinically significant weight gains.1
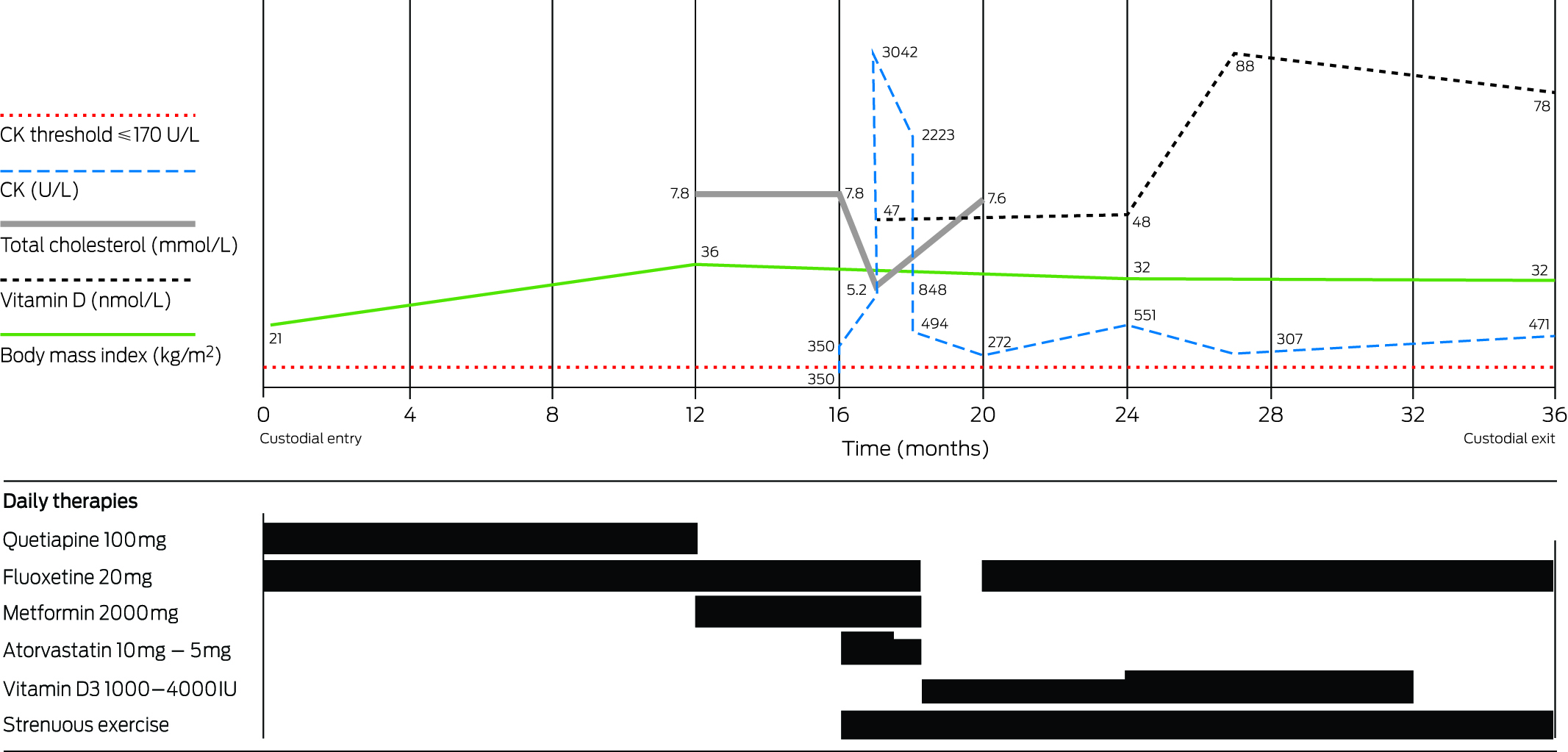
Creatine kinase (CK) and other markers according to time and medications.
Provenance: Not commissioned; externally peer reviewed.
- Leigh Haysom1
- Katherine Samaras2,3
- Catherine Stapylton1
- Jennifer Wines4
- 1 Justice Health and Forensic Mental Health Network, Sydney, NSW.
- 2 Garvan Institute of Medical Research, Sydney, NSW.
- 3 St Vincent's Hospital, Sydney, NSW.
- 4 Justice Health and Forensic Mental Health Network, Wagga Wagga, NSW.
No relevant disclosures.
- 1. Curtis J, Newall H, Myles N, et al. Considering metformin in cardiometabolic protection in psychosis. Acta Psychiatr Scand 2012; 126: 302-303.
- 2. Anderson P, Bhatia K, Cunningham J. Mortality of Indigenous Australians. Canberra: Australian Bureau of Statistics, 1996 (ABS Occasional Paper No. 3315.0.) http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/05FDC61976A736D7CA2568C40005CF5A (accessed Nov 2014).
- 3. Medicare Australia. Closing the gap — PBS co-payment measure. www.medicareaustralia.gov.au/provider/pbs/prescriber/closing-the-gap.jsp (accessed Apr 2014).
- 4. Gabb GM, Vutry A, Limaye V, Alhami G. Serious statin-associated myotoxicity and rhabdomyolysis in Aboriginal and Torres Strait Islanders: a case series. Intern Med J 2013; 43: 987-992.
- 5. Bastian I, Dent J, McFarlane R, et al. HTLV-I among Northern Territory blood donors. Med J Aust 1993; 159: 7-12.
- 6. Santos PC, Soares RA, Nascimento RM, et al. SLCO1B1 rs4149056 polymorphism associated with stain-induced myopathy is differently distributed according to ethnicity in the Brazilian general population: Amerindians as a high risk ethnic group. BMC Med Genet 2011; 12: 136.
- 7. László A, Kalabay L, Nemcsik J. Case report of exercise and statin-fibrate combination therapy-caused myopathy in a patient with metabolic syndrome: contradictions between the two main therapeutic pathways. BMC Res Notes 2013; 6: 52.
- 8. McKelvie PA, Dennett X. Myopathy associated with HMG-CoA reductase inhibitors (statins): a series of 10 patients and review of the literature. J Clin Neuromuscul Dis 2002; 3: 143-148.
- 9. Padala S, Thompson PD. Statins as a possible cause of inflammatory and necrotizing myopathies. Atherosclerosis 2012; 222: 15-21.
- 10. Indig D, Topp L, Ross B, et al. 2009 NSW Inmate Health Survey: key findings report. Sydney: Justice Health, 2010.
- 11. International Physical Health in Youth Stream (iphYs) Working Group. Healthy Active Lives (HeAL): keeping the body in mind in youth with psychosis [consensus statement] 2013.





Genevieve MaryGabb
However, it is important to understand the suggestion Indigenous Australians may be at higher risk of statin-associated myotoxicity is a hypothesis only, due to uncertainty about number of cases, and limitations of medicines use data. The role of Vitamin D deficiency and/or human T-cell lymphotropic virus type I infection in the aetiology of the condition is also uncertain.
Genetic variations in SLCO1B1 gene are associated with responses to simvastatin, rather than all statins as a drug class (3). As most cases of statin-associated myotoxicity in Aboriginal and Torres Strait Islanders have occurred with atorvastatin (presumably reflecting patterns of drug use), there is no clear relevance of this genetic variation to statin myotoxicity in Indigenous Australians.
In the face of these uncertainties, there are some things which are known to be true, or can be logically deduced. These include: effective medications have adverse effects; adverse effects vary with racial status (4); drug development programs often do not include Indigenous Australians; safety of medicine in Aboriginal and Torres Strait Islanders is uncertain.
The precautionary principle suggests it is the responsibility of the proponents of an activity to establish the proposed activity will not result in significant harm. In this context, perhaps the ‘perfect storm’ was the establishment of the Indigenous Chronic Disease Package, without a companion pharmacovigilance and risk mitigation policy and program, rather than the individual circumstances of the patient described. The same can be said of drug safety for Aboriginal and Torres Strait Islanders, as was said previously in relation to pharmacovigilance generally, ‘it is not enough to be content with an absence of evidence on harms, but we need to move to a position where we have evidence of absence of harm.’ (5)
1. Gabb GM, Vitry A, Limaye V, Alhami G. Serious statin-associated myotoxicity and rhabdomyolysis in Aboriginal and Torres Strait Islanders: a case series. Int Med J 2013;43:987-992
2. Wood J, Robertson T, Pui K, Ly J, Roberts L, Fayez S, Lakshman U, Soden M. Statin-associated necrotizing autoimmune myopathies in the Indigenous population: a case series from North Queensland. Int Med J 2015;45:s20
3. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsude F et al. SLCO1B1 variants and statin-induced myopathy – a genomewide study. N Engl J Med 2008;359:789-99
4. Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharm Ther 2015;97:263-273
5. http://ec.europa.eu/health/files/pharmacovigilance/docs/2007_02_26/19.pdf
Competing Interests: No relevant disclosures
Dr Genevieve MaryGabb
SA Health