Invasive pneumococcal disease (IPD) is a major cause of morbidity in very young children and older adults.1 The 23-valent polysaccharide pneumococcal vaccine (23vPPV) has been available in Australia since 1986, and use of it has increased progressively since then. It was recommended and subsidised under the Pharmaceutical Benefits Scheme for Australians aged ≥ 65 years in 1997, provided free of charge for this group in Victoria from 1998, and included in the nationally funded National Immunisation Program from 2005.2 The vaccine’s effectiveness against IPD in immunocompetent older people has been estimated as about 70%,3 but it is generally regarded as not effective in preventing carriage of pneumococcal serotypes against which it is targeted.1,3
Australia is the only country to have introduced nationally funded programs for the 7-valent pneumococcal conjugate vaccine (7vPCV) for infants and the 23vPPV for older people in the same year (2005).4 Unlike the 23vPPV, the 7vPCV has been shown to prevent carriage of vaccine serotypes,1 resulting in herd immunity impacts. Reductions in IPD due to 7vPCV serotypes, in vaccinated and unvaccinated age groups, have been observed in many countries. Increases in non-7vPCV serotypes (referred to as serotype replacement) have also been observed in vaccinated and unvaccinated age groups, with the net impact on total IPD incidence varying from country to country.5
In Victoria, a 36% decrease in IPD incidence occurred in people over 65 years of age following the introduction of funded 23vPPV in that state in 1998;6 this was before widespread use of the 7vPCV in children. 7vPCV coverage among infants rose rapidly to 90% in Australia in the first year of the nationally funded program.7 As all 7vPCV serotypes are also present in 23vPPV, any herd immunity effect from 7vPCV would complicate interpretation of the impact of 23vPPV in older people. Studies from two regions on IPD incidence in Australian adults in the post-7vPCV era have provided conflicting evidence on the changes in total IPD incidence in older people.8,9
In July 2011, the 13-valent pneumococcal conjugate vaccine (13vPCV) was introduced for all Australian infants, replacing the 7vPCV and (in the Northern Territory) the 10-valent pneumococcal conjugate vaccine.10 Unlike the 7vPCV, the 13vPCV has been licensed for use in adults aged ≥ 50 years in the United States and Australia, but has not yet been included in the National Immunisation Program.
This study was exempt from the requirement for ethics approval as it was conducted as a quality assurance exercise pertaining to the National Immunisation Program, under the auspices of the Australian Technical Advisory Group on Immunisation. De-identified data were provided for this purpose by the Communicable Diseases Network Australia.
Population rates of IPD by serotype category in people aged ≥ 65 years by year from 1 January 2002 to 31 December 2011 were plotted with 95% confidence intervals, which were calculated using the Poisson distribution of notification numbers. The estimated residential populations, minus Indigenous population estimates from the 2006 Australian census, were used as denominators for calculations of rates in the non-Indigenous population.11
Vaccination coverage estimates for people aged ≥ 65 years, by jurisdiction, were available from adult vaccination surveys conducted by the Australian Institute of Health and Welfare in 2004, 2006 and 2009.12-14 These were based on respondents’ report in telephone interviews regarding receipt of pneumococcal or pneumonia vaccine within the previous 5 years, without reference to written vaccination records.
Changes in rates from the pre-7vPCV era (2002–2004) to the recent post-7vPCV era (2010–2011) periods by serotype category were measured using incidence rate ratios (IRRs) with 95% confidence intervals. IRRs for the vaccinated age group (≥ 65 years) were compared with those for the unvaccinated age group (50–64 years), in which 23vPPV coverage was low (< 5%).7
Estimates of vaccine effectiveness (VE) in the ≥ 65-year age group were calculated using the screening method, a form of case–cohort study. It compares the likelihood of prior vaccination in 23vPPV serotype IPD cases with that for the total population. It uses the formula VE=1−[CV÷(1−CV)]×[(1−PV)÷PV], where CV is the proportion of cases that occurred in vaccinated people, and PV is the proportion of the population that had been vaccinated.15
The proportion of the population vaccinated was obtained from the adult vaccination surveys conducted in 2004, 2006 and 2009.12-14 Separate estimates for the 65–74-year and ≥ 75-year age groups by jurisdiction were available from the 2004 and 2006 surveys.
A logistic regression model was fitted using the GENMOD procedure in SAS 9.1 (SAS Institute) as described previously.16 Data were stratified by year, age group and, for 2004 and 2006, by jurisdiction. A sensitivity analysis of the VE estimates was conducted, recalculating VE using a deviance of ± 10% of the total population in coverage estimates. Statistical analysis was carried out in SAS 9.1.3. Statistical significance was established by non-overlapping 95% confidence intervals, of rates and rate ratios.
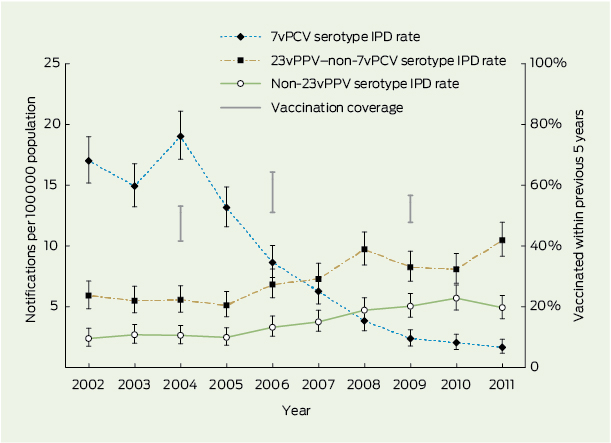
Annual IPD notification rates in people aged ≥ 65 years by serotype category are shown in Box 1. There was a substantial and statistically significant decrease in 7vPCV serotype IPD during the post-7vPCV era (2005–2011), as well as significant increases in 23vPPV–non-7vPCV and non-23vPPV serotypes, based on non-overlapping confidence intervals of annual rates.
The range of self-reported vaccination coverage (percentage vaccinated in the previous 5 years) estimates for those aged ≥ 65 years are also shown in Box 1, for all jurisdictions except Victoria. Coverage ranged from 41% to 53% in individual jurisdictions in 2004, increased to 51%–64% in 2006 and decreased to 48%–56% in 2009.
Pre-7vPCV (2002–2004) to post-7vPCV (2010–2011) changes in IPD rates in the ≥ 65-year age group and the 50–64-year age group are shown in Box 2. In both age groups there were substantial, statistically significant decreases for 7vPCV serotypes and increases for 23vPPV-non-7vPCV and non-23vPPV serotypes, based on IRRs not overlapping 1.0. The magnitude of these changes did not differ significantly between the two age groups. For all serotypes, the IRR point estimate was lower in the ≥ 65-year age group, but confidence intervals for the two age groups overlapped.
Numbers of IPD cases and VE estimates for 23vPPV against 23vPPV-type IPD are shown in Box 3. All VE estimates were statistically significantly above zero. The point estimate for 2009 was lower than for 2004, but was compatible with it, as confidence intervals overlapped. A sensitivity analysis to evaluate the impact of varying population coverage estimates for 23vPPV yielded an upper VE estimate of 75.8% (95% CI, 72.1%–79.9%) if true population coverage was 10% higher than estimated in the adult vaccination survey and a lower VE estimate of 40.5% (95% CI, 31.1%–49.9%) if true population coverage was 10% lower than estimated.
Changes in IPD rates over time by serotype category presented here provide evidence of a substantial herd immunity impact in older people due to 7vPCV use in infants. However, an impact on IPD rates directly resulting from 23vPPV use in older people, by comparing changes in vaccinated and unvaccinated age groups, was not clearly shown. The VE estimate for 23vPPV against 23vPPV-type IPD was 61.1%. An overall decrease of 35% was observed in total IPD rates in ≥ 65-year-olds 6–7 years after the commencement of the nationally funded programs for 7vPCV and 23vPPV (25.2 notifications/100 000 population/year in 2002–2004 v 16.4 in 2010–2011).
Herd immunity impacts in adults from use of the 7vPCV in children have been shown in many countries. Herd immunity impacts on total IPD rates are heavily dependent on the pre-vaccination serotype distribution in adults and the length of time since 7vPCV introduction, as serotype replacement increases over time.5 Australian non-Indigenous people had one of the highest proportions of 7vPCV-type IPD out of total IPD in the world, similar to that in the US, and these are the only two countries with net decreases in IPD reported for older people following 7vPCV introduction.17
Trends in IPD rates by year in Australia in our study did not show clear evidence of a reduction of disease incidence due to use of 23vPPV in people aged ≥ 65 years. However interpretation of this finding is complicated by two factors: an overall modest level of 23vPPV coverage and a relatively small increase in coverage after national funding began in 2005; and the apparent indirect effects of introducing the 7vPCV for infants at the same time. The comparison of rates in vaccinated and unvaccinated age groups, both subject to herd immunity impacts from infant vaccination, allows the possibility of some impact from the 23vPPV. The absence of impacts on population IPD rates following publicly funded 23vPPV for ≥ 65-year-olds has also been reported in the US18 and United Kingdom.19 Gradual increases in coverage also occurred in those settings, and formal VE assessments in adults have consistently shown significant VE.20-22
All observational methods used to estimate VE are subject to bias. For our application of the screening method, different methods were used to ascertain the vaccination status of the general population (telephone survey) and the vaccination status of 23vPPV-type IPD cases (general practitioner and/or patient interview). However, our sensitivity analysis showed that the VE estimate remained statistically significant even if the true population coverage was 10% lower than the adult vaccination survey estimates. A study of 23vPPV vaccination status in older people in Victoria found that patient recall underestimated vaccination status by 6% compared with medical records.23
During the period of our study, a single revaccination was recommended for people first vaccinated at ≥ 65 years of age. As of December 2011, this is no longer recommended.10 The latest national estimate of the proportion of people aged ≥ 65 years who have ever received 23vPPV is a modest 59%.13 Given the evidence of the vaccine’s effectiveness, higher coverage would be expected to increase the impact of the vaccine in reducing IPD incidence.
The appeal of a conjugate vaccine used in older people includes potential, although unproven, benefits such as a superior response to booster doses and impacts on carriage and non-invasive pneumonia. Herd immunity impacts of 7vPCV on non-invasive pneumonia in older people have been reported as being non-existent, very small or extensive.24-26 A randomised controlled trial assessing the impact of 13vPCV use in older people on pneumonia is currently underway.27 However, this trial is not being conducted alongside concurrent use of 13vPCV in infants. The incremental benefits of 13vPCV use in older people in addition to an infant program would be more difficult to evaluate.
1 IPD notification rates by serotype category for ≥ 65-year-old Australians and pneumococcal vaccination coverage, 2002–2011*
2 Invasive pneumococcal disease notifications and notification rates for unvaccinated (50–64 years) and vaccinated (≥ 65 years) age groups of older Australians by serotype group, 2002–2004 versus 2010–2011*
Notifications per 100 000 population per year |
|||||||||||||||
Received 2 December 2012, accepted 11 December 2013
- Robert I Menzies1,2
- Sanjay H Jayasinghe1
- Vicki L Krause3
- Clayton K Chiu2
- Peter B McIntyre2
- 1 National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases, Sydney, NSW.
- 2 Sydney Medical School, University of Sydney, Sydney, NSW.
- 3 Centre for Disease Control, Department of Health, Darwin, NT.
We thank members of the Pneumococcal Working Party of the Australian Technical Advisory Group on Immunisation, for whom this work was done, for providing comments on methods and interpretation (Kim Mulholland, Ross Andrews, Peter Richmond, Raina MacIntyre, Sue Campbell-Lloyd, Jonathan Carapetis, Lyn Gilbert, Chris Blyth, Stephen Wesselingh) and the Enhanced Invasive Pneumococcal Disease Surveillance Working Group of the Communicable Diseases Network Australia for collecting and providing the IPD data (Heather Cook, Christina Bareja, Robin Gilmour, Hannah Vogt, Lucinda Franklin, Carolien Giele, Angela Wakefield, Helen Smith, Geoffrey Hogg, Sue Reid, David Coleman, Shahin Oftadeh, Janet Strachan, Lyn Gilbert, Rob Menzies, Vicki Krause, Stacey Rowe). We also thank Mary Sinclair for helping to prepare the manuscript. The Australian Institute of Health and Welfare provided unpublished data from adult vaccination surveys. The National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases is supported by the Australian Government Department of Health and Ageing, the New South Wales Ministry of Health and the Sydney Children’s Hospitals Network.
The National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases was supported to conduct this study, and the manuscript approved, by the Australian Government Department of Health and Ageing. However, the views expressed are not necessarily those of the Department.
- 1. Black S, Eskola J, Whitney C, Shinefield H. Pneumococcal conjugate vaccine and pneumococcal common protein vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: WB Saunders, 2008: 531-567.
- 2. National Health and Medical Research Council. The Australian immunisation handbook. 9th ed. Canberra: Australian Government Department of Health and Ageing, 2008.
- 3. Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; (1): CD000422.
- 4. Australian Government Department of Health and Ageing. New: universal childhood pneumococcal vaccination program. 2004. http://www.immunise. health.gov.au/pneumococcal/index.htm (accessed Jul 2009).
- 5. Feikin D. Review of serotype replacement in the setting of PCV7 use and implications for the PCV10/PCV13 era. Strategic Advisory Group of Experts (SAGE) on Immunization, World Health Organization, 2011. http://www.who.int/immunization/sage/SAGEReplacementReport2011FINAL_nov11.pdf (accessed Jan 2014).
- 6. Andrews RM, Counahan ML, Hogg GG, McIntyre PB. Effectiveness of a publicly funded pneumococcal vaccination program against invasive pneumococcal disease among the elderly in Victoria, Australia. Vaccine 2004; 23: 132-138.
- 7. Menzies R, Turnour C, Chiu C, McIntyre P. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia, 2003 to 2006. Commun Dis Intell Q Rep 2008; 32 Suppl: S2-S67.
- 8. Lehmann D, Willis J, Moore HC, et al. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis 2010; 50: 1477-1486.
- 9. Hanna JN, Humphreys JL, Murphy DM, Smith HV. Invasive pneumococcal disease in non-Indigenous people in north Queensland, 2001–2009. Med J Aust 2010; 193: 392-396. <MJA full text>
- 10. Australian Government Department of Health. Pneumococcal disease. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-pneumococcal (accessed Nov 2013).
- 11. Australian Bureau of Statistics. Population projections, Australia, 2006 to 2101. (ABS Cat. No. 3222.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3222.02006%20to%202101?OpenDocument (accessed Dec 2013).
- 12. Australian Institute of Health and Welfare. 2004 adult vaccination survey: summary results. Canberra: AIHW, 2005. (AIHW Cat. No. PHE 56.) http://www.aihw.gov.au/publication-detail/?id=6442467710 (accessed Dec 2013).
- 13. Australian Institute of Health and Welfare. 2009 adult vaccination survey: summary results. Canberra: AIHW, 2011. (AIHW Cat. No. PHE 135.) http://www. aihw.gov.au/publication-detail/?id=10737418409 (accessed Dec 2013).
- 14. Australian Government Department of Health and Ageing. 2006 adult vaccination survey: coverage and valid usage — summary results. Canberra: DOHA, 2008. http://www.health.gov.au/internet/immunise/publishing.nsf/Content/AVS-cnt/$File/2006-avs.pdf (accessed Dec 2013).
- 15. Torvaldsen S, McIntyre PB. Observational methods in epidemiologic assessment of vaccine effectiveness. Commun Dis Intell Q Rep 2002; 26: 451-457.
- 16. Torvaldsen S, Simpson JM, McIntyre PB. Effectiveness of pertussis vaccination in New South Wales, Australia, 1996–1998. Eur J Epidemiol 2003; 18: 63-69.
- 17. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein–polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737-1746.
- 18. Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease — United States, 1998–2003. MMWR Morb Mortal Wkly Rep 2005; 54: 893-897.
- 19. Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11: 760-768.
- 20. Andrews NJ, Waight PA, George RC, et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012; 30: 6802-6808.
- 21. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325: 1453-1460.
- 22. Vila-Córcoles A, Ochoa-Gondar O, Hospital I, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43: 860-868.
- 23. Andrews RM. Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia. Vaccine 2005; 23: 2756-2761.
- 24. Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J 2010; 29: 607-612.
- 25. Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369: 1179-1186.
- 26. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369: 155-163.
- 27. Hak E, Grobbee DE, Sanders EA, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med 2008; 66: 378-383.





Abstract
Objective: To evaluate the impact and effectiveness of the 23-valent polysaccharide pneumococcal vaccine (23vPPV) in ≥ 65-year-old Australians in the context of concurrent 7-valent pneumococcal conjugate vaccine (7vPCV) use in infants.
Design, patients and setting: Ecological analysis of trends in invasive pneumococcal disease (IPD) notification rates and vaccine effectiveness estimation using the screening method, using data on Australians aged ≥ 65 years (23vPPV funded) and 50–64 years (23vPPV not funded).
Intervention: National 23vPPV program for people aged ≥ 65 years and national 7vPCV program for infants, both commencing in 2005.
Main outcome measures: IPD incidence rate ratios, 2002–2004 to 2010–2011, and 23vPPV effectiveness against 23vPPV-type IPD.
Results: The proportion of people aged ≥ 65 years who were vaccinated within the previous 5 years in jurisdictions excluding Victoria ranged from 41% to 64% over the study period, with no clear trend over time. Incidence rate ratios in the ≥ 65-year age group were 0.11 (95% CI, 0.09–0.14) for 7vPCV serotypes, 1.64 (95% CI, 1.41–1.91) for 23vPPV–non-7vPCV serotypes and 2.07 (95% CI, 1.67–2.57) for non-23vPPV serotypes. The incidence rate ratio for total IPD was 0.65 (95% CI, 0.59–0.71) for people aged ≥ 65 years, and 0.80 (0.71–0.90) for people aged 50–64 years. The estimate of 23vPPV effectiveness was 61.1% (95% CI, 55.1%–66.9%).
Conclusions: The greater reduction in IPD among ≥ 65-year-olds compared with 50–64-year-olds did not reach statistical significance. However, vaccine effectiveness was significant. Greater reductions in IPD in ≥ 65-year-olds would be expected from the indirect effects of using 13-valent pneumococcal conjugate vaccine in infants (introduced for Australian infants in 2011) and an increase in 23vPPV coverage.