Clinical record
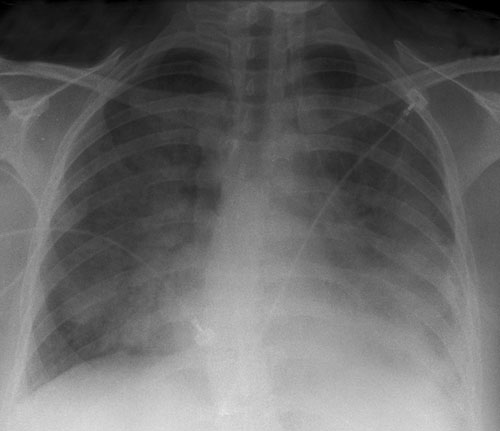
On Day 2, the patient continued to be febrile and two sets of blood cultures were performed. Further tests showed a raised C-reactive protein level (380 mg/L; RI, < 8.0 mg/L) and a mild coagulopathy (international normalised ratio, 1.4; RI, 0.8–1.2) with thrombocytopenia (platelet count, 123 × 109/L; RI, 150–400 × 109/L) suggesting early disseminated intravascular coagulation. That evening, the patient developed respiratory distress and hypoxaemia, with an arterial partial pressure of oxygen of 66 mmHg despite receiving a fraction of inspired oxygen (Fio2) of 80%. She was transferred to the intensive care unit, where she received non-invasive ventilation. A chest x-ray revealed bilateral patchy infiltrates consistent with a pneumonic process (Box 1). Antibiotic treatment with ceftriaxone and azithromycin was initiated.
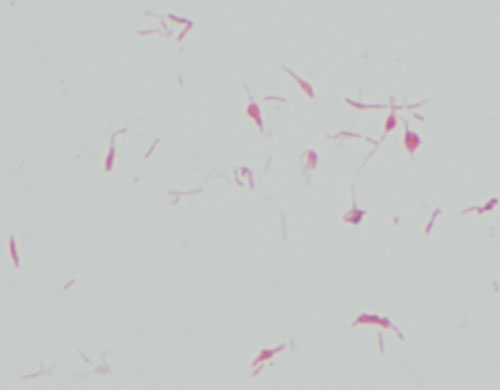
On Day 3, growth was detected in the aerobic bottle from one set of blood cultures after 20 hours of incubation (BACTEC Plus, BD Diagnostics, Sparks, Md, USA), and antibiotic treatment was changed to piperacillin–tazobactam and ciprofloxacin. An initial Gram stain showed a thin gram-negative rod with no distinguishing features. After 24 hours of incubation, light growth of an organism was noted on blood and chocolate agars (Oxoid Australia, Thebarton, SA) which had been incubated anaerobically and in supplemental carbon dioxide. Gram stain of these colonies showed irregular, bulbous gram-negative organisms typical of Streptobacillus moniliformis (Box 2).
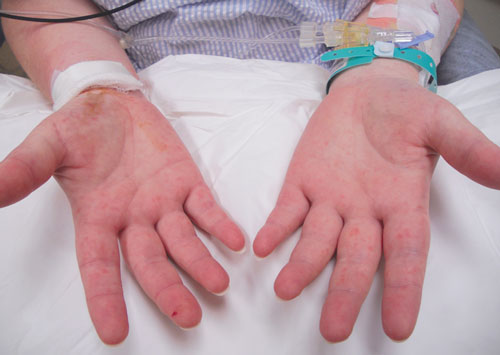
On Day 5, a blanching macular rash involving all limbs, including palms and soles, became apparent (Box 3). Histological examination of a biopsy sample of a macule from the patient’s hand showed a neutrophilic inflammation of the small dermal vessels consistent with leukocytoclastic vasculitis. By this time, the patient’s respiratory function had improved markedly and she was transferred to a medical ward.
This case highlights the importance of history-taking and the need to perform blood cultures in patients presenting with fever. The differential diagnoses considered were wide and included bacterial sepsis, viral infection and autoimmune disease. Bacterial sepsis from streptococcal, meningococcal or staphylococcal infection was considered most likely in this patient. Definitive diagnosis was helped by the isolation of S. moniliformis from blood culture, which allowed targeted therapy and improved prognosis.
It is possible that our patient was co-infected with an arenavirus such as lymphocytic choriomeningitis virus (LCMV). Although LCMV is rare, it can be acquired by handling pet rodents or their excreta and can cause a flu-like illness with aseptic meningitis.1 Diagnosis of LCMV infection would require molecular testing of blood or CSF in a reference laboratory.
S. moniliformis, a causative agent of rat bite fever, is part of the commensal flora of the rat’s oropharynx.2 Another form of rat bite fever — known as “sodoku” — is caused by Spirillum minus, a spirochaete-like organism.2 Sodoku generally differs from S. moniliformis infection by causing induration at the site of the rodent bite and having an incubation period of more than 10 days. The incubation period for S. moniliformis infection is typically fewer than 7 days.2
Rat bite fever, as the name suggests, is usually acquired through a rat bite. However, the disease can result from handling and exposure to excreta or saliva of rodents such as rats or guinea pigs.2,3 S. moniliformis infection can also be caused by ingestion of contaminated milk; in such cases it is known as Haverhill fever, as the first known outbreak (in 1926) occurred in Haverhill, Massachusetts.2
Clinical manifestations of S. moniliformis infection include fever, headache, rash and polyarthritis. The rash typically involves the extremities, including the palms and soles, with a leukocytoclastic vasculitis seen on histology.4 The disease is often mild or self-limiting. However, infection is potentially lethal and can cause rapid death in previously healthy adults.2,5 The overall mortality rate is estimated to be 13%.2 Although less frequently reported than fever, headache, rash and polyarthritis, pneumonitis and endocarditis are common autopsy findings in those who succumb to the disease.5-7 Meningitis due to S. moniliformis is rare, but it has been described in Dutch and Portuguese literature.8,9
The standard recommended therapy is penicillin, but S. moniliformis is susceptible to a number of other antibiotics including cephalosporins and macrolides. In cases where the organism has been isolated, in-vitro antibiotic susceptibility testing should be done for any antibiotic being considered for clinical use.2
Systemic illness following rat bite has been recognised for thousands of years. Historically, the disease was associated with squalor and slum-dwelling. However, victims in recent years have included laboratory workers, pet shop employees and, increasingly, owners of pet rats.2,4,5,10 As this case demonstrates, a bite is not necessary for infection — close contact with rodents may be sufficient. As rodents become more popular as household pets, more cases of S. moniliformis infection due to affectionate contact are likely to occur.
1 Chest radiograph (mobile, erect) taken on Day 2, showing diffuse bilateral infiltrates consistent with a pneumonic process
2 Gram stain of Streptobacillus moniliformis cultured from the patient’s blood (100 × magnification)
Lessons from practice
Rat bite fever caused by Streptobacillus moniliformis is an uncommon but potentially lethal systemic infection.
The organism has a distinctive Gram stain appearance which allows for early presumptive identification.
A thorough history, including history of animal exposure and pet ownership, should be taken for all febrile patients.
Transmission of S. moniliformis can occur by affectionate contact; a bite is not required.
- 1. Centers for Disease Control and Prevention. Interim guidance for minimizing risk for human lymphocytic choriomeningitis virus infection associated with rodents. MMWR Morb Mortal Wkly Rep 2005; 54: 747-749.
- 2. Elliott SP. Rat bite fever and Steptobacillus moniliformis. Clin Microbiol Rev 2007; 20: 13-22.
- 3. Fordham JN, McKay-Ferguson E, Davies A, Blyth T. Rat bite fever without the bite. Ann Rheum Dis 1992; 51: 411-412.
- 4. Tandon R, Lee M, Curran E, et al. A 26-year-old woman with a rash on her extremities. Clin Infect Dis 2006; 43: 1585-1586.
- 5. Centers for Disease Control and Prevention. Fatal rat-bite fever — Florida and Washington, 2003. MMWR Morb Mortal Wkly Rep 2005; 53: 1198-1202.
- 6. McHugh TP, Bartlett RL, Raymond JI. Rat bite fever: report of a fatal case. Ann Emerg Med 1985; 14: 1116.
- 7. Sens MA, Brown EW, Wilson LR, Crocker TP. Fatal Streptobacillus moniliformis infection in a two-month old infant. Am J Clin Pathol 1989; 91: 612.
- 8. Beeuwkes H, De Jong JG, Van Der Zalm HO. [Cranial base fracture with purulent meningitis due to Streptobacillus moniliformis] [Dutch]. Ned Tijdschr Geneeskd 1954; 98: 906-912.
- 9. Atala A, Correa CN, Correa WM, et al. [Meningitis caused by Streptobacillus moniliformis] [Portuguese]. Rev Paul Med 1973; 82: 175-178.
- 10. Sakalkale R, Mansell C, Whalley D, et al. Rat-bite fever: a cautionary tale. N Z Med J 2007; 120: U2545.





No relevant disclosures.