Gentamicin is an important bactericidal antibiotic with two serious potential adverse effects: nephrotoxicity and ototoxicity. Clinicians are well aware that rising serum creatinine levels in patients treated with gentamicin could indicate nephrotoxicity. However, many do not know that, contrary to textbooks and antibiotic guidelines, gentamicin ototoxicity causes impairment of vestibular, not auditory, function.1,2 Vestibulotoxicity is frequently overlooked in patients having gentamicin,3-7 so that severe, irreversible, bilateral vestibular loss can occur, causing permanent imbalance, which is particularly debilitating in elderly people.
Over the 23-year period, 552 patients were diagnosed with severe, symmetrical, selective (ie, normal hearing for age), bilateral vestibular loss. Of these, 263 patients had gentamicin vestibulotoxicity (GVT). In the remainder, the vestibular loss was caused by cisplatinum ototoxicity (9), meningitis (6), hereditary factors (29), or bilateral sequential vestibular neuritis (61); in 184 the condition was idiopathic.8
Ethics approval was not required for this retrospective review.
Bilateral vestibular loss was diagnosed if all the following gave positive results: (i) bi-directional, horizontal and vertical head impulse test (Video 1, Video 2, Video 3, Video 4);9 (ii) vertical oscillopsia with loss of at least three lines of visual acuity on a Snellen chart during vertical head shaking (Video 5);10 and (iii) negative results of a Romberg test on a firm surface and positive results on a foam surface (Video 6).11
To confirm the diagnosis and quantify vestibular loss, 95 patients with GVT (the remaining eight were too frail to test) had either caloric or rotational vestibular testing of lateral semicircular canal function, or both (Box 1).12
Air-conduction, pure-tone threshold, clinical audiometric graphs (0.25–8 kHz), from hearing measurement in each ear when GVT was diagnosed, were available in 73 of the 103 patients. Frequencies above 8 kHz were not tested. In patients with air-conduction thresholds above normal for age and in those with middle-ear disease, bone-conduction thresholds were also measured. Audiologists’ descriptive reports of the audiometric results were available for the remaining 30 patients.
We reviewed patients’ clinical diagnoses to determine (i) whether gentamicin therapy complied with contemporary (at the time of admission to hospital) or with current (2010) Australian antibiotic guidelines;13 and (ii) whether the treatment was empirical, but appropriate, or based on results of cultures.
The 103 patients who fulfilled our criteria comprised 47 men, 56 women; mean age, 64 years (range, 18–84 years). Forty-eight presented with both imbalance and oscillopsia, 39 with imbalance only, and four with oscillopsia only; and in 12 patients we were unable to determine their main presenting symptom. In all patients, the following tests gave positive results: a bilateral clinical head impulse test,9 a vertical head-shaking test for vertical oscillopsia,10 and a foam Romberg test (Video 1, Video 2, Video 3, Video 4, Video 5, Video 6).11 Thirty-eight patients had recurrent falls or required a walking aid, and 44 required vestibular rehabilitation.14
The delay to diagnosis of bilateral vestibular loss ranged from 4 days (the only patient in whom GVT was diagnosed during treatment) to 15 years. GVT was diagnosed less than 12 months after treatment in 69 patients, and more than 12 months after treatment in 34 patients.
Only three of the 103 patients with GVT complained of hearing impairment after gentamicin treatment, in each case while still in hospital. No patient had audiometry at the time gentamicin was given, but all had audiometry later as part of the assessment of vestibular loss. For the 73 patients whose audiograms were retrieved, hearing loss at each frequency, averaged for each age group and both ears, was not different from accepted age-group means (Box 2).15,16 Audiometry in the three patients who complained of hearing loss also showed thresholds consistent with age. The pure-tone thresholds in the 30 descriptive reports were reported as normal or showing only high-frequency hearing loss, consistent with age and noise exposure. We were unable to retrieve audiometry data from before gentamicin treatment for any of the patients.
During gentamicin treatment 43 of 103 patients developed nephrotoxicity, defined as a serum creatinine level > 120 µmol/L (range, 121–841 µmol/L) and eGFR < 60 mL/min/1.73 m2 (range, 59–< 6 mL/min/1.73 m2) on two sequential daily measurements.
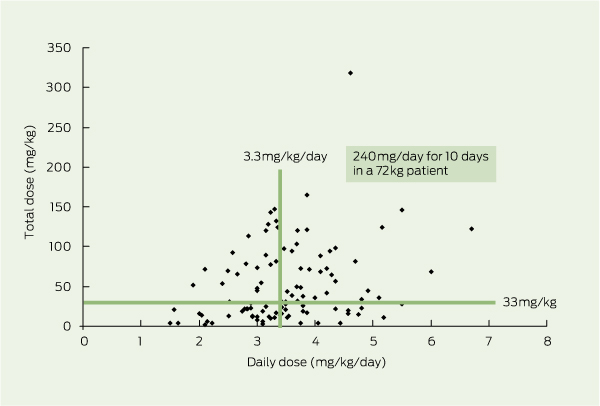
The gentamicin dose ranged from 160 to 320 mg/day, equivalent to 1.5 to 5.6 mg/kg/day (mean, 3.5 mg/kg/day). The total gentamicin dose ranged from 160 to 16 520 mg, equivalent to 2– 318 mg/kg (mean, 3639 mg or 52 mg/kg). The mean total dose in the 43 patients who developed nephrotoxicity was 4363 mg, in contrast to 3240 mg in the 60 who did not. The gentamicin doses our patients with GVT received in hospital, between 1975 and 2010, indicate that there was no change in the total dose or daily dose of gentamicin given in this period. Box 3 shows total dose (mg/kg) versus daily dose (mg/kg/day) for each patient.
Duration of treatment ranged from 1 day (the six patients who had only a single dose) to 80 days (mean, 17 days).
It was not possible from the treatment chart information to determine the exact method of intravenous administration used: slow infusion (recommended) or bolus injection.
Serum gentamicin levels (trough or peak or both) were retrieved in 82 patients; in 23, a trough or a peak level was above the recommended range. Peak levels were higher in those who developed nephrotoxicity than in those who did not (Box 4).
Adherence to contemporary or current antibiotic guidelines for gentamicin use13 is shown by patient diagnosis in Box 5 and by clinician subspecialty in Box 6.
All these patients developed symptoms and signs of bilateral vestibular impairment after treatment with gentamicin, but only three noted any hearing impairment. Audiometric thresholds in the all patients were not significantly different from age-matched thresholds.15,16 Unless pre-gentamicin audiometry is available, high-frequency (4–8 kHz) hearing loss, especially in elderly men, cannot be assumed to be due to gentamicin; noise and ageing are much more common causes of hearing loss.1 Measuring hearing before and after gentamicin treatment17,18 shows only slight (about 15 dB) high-frequency (4–8 kHz), asymptomatic hearing loss. This suggests that any hearing loss from gentamicin ototoxicity would not be noticed by patients with normal hearing,19,20 and certainly not by those with pre-existing, high-frequency hearing loss. By contrast, symptoms of vestibular loss are obvious to patients, as are the clinical signs to the aware clinician.
All patients noted imbalance either during hospital admission or immediately after discharge. We were able to confirm that they had received gentamicin and had no other potential cause for bilateral vestibular loss. Even though it has been known since 195221 that certain aminoglycosides, including streptomycin and gentamicin, can cause severe, selective loss of vestibular function,1-8 prescribers rarely recognise GVT.3,4
Because vestibular loss is, at least initially, bilateral and symmetrical, patients will develop imbalance and oscillopsia, not vertigo, the hallmark of acute, unilateral vestibular loss. Bed-bound patients will be unaware of their imbalance until they walk again. Unless clinicians know how to test for bilateral vestibular loss, gentamicin treatment will not be stopped and vestibulotoxicity will most likely be aggravated. Bilateral vestibular loss can now be objectively confirmed at the bedside using video-vestibulometry (Box 7; Video 3, Video 4),22 dynamic visual acuity10 and the foam Romberg test (in ambulant patients) (Video 6).11
Even when patients report that imbalance developed after hospital admission, gentamicin is rarely considered as the possible cause. With delayed diagnosis, it is difficult without a subpoena23 to obtain hospital drug treatment charts to confirm that gentamicin was given.
There has not been any change in the overall gentamicin dose given to patients who develop GVT since 1994.3 We found that regardless of total dose, daily dose, number of doses per day or duration of treatment, patients can still develop GVT. We also confirmed that GVT can occur even when serum trough or peak levels are at or below the recommended range.3,4
A study of 33 patients with GVT also found no relationship between daily dose, total dose or serum levels and GVT, and 32 of their patients were diagnosed after hospital discharge.4
The mean total dose and serum levels of gentamicin in the 43 patients with GVT who developed nephrotoxicity24,25 were substantially higher than in the 60 who did not. Patients who develop vestibulotoxicity seem more likely to develop nephrotoxicity (42%) than patients in general receiving gentamicin (5%–17%),26 suggesting a common mechanism or predisposing factor.
The current (2010) Australian antibiotic guidelines advise that use of gentamicin for empirical treatment should be limited to 48 hours, pending the results of microbiological investigations. Directed therapy is indicated only for infections in which there is resistance to other, safer, antimicrobials; for combination therapy in serious Pseudomonas aeruginosa and Brucella infections; and as synergistic treatment for streptococcal or enterococcal endocarditis. It should be considered for prophylactic use only in patients at specific risk of developing endocarditis from genitourinary or gastrointestional procedures.13
Gentamicin was given according to current antibiotic guidelines13 in 47% of our 103 patients; 52% were given empirical gentamicin for longer than the recommended 48 hours; and in only 46% of patients was gentamicin given in accordance with contemporary antibiotic guidelines. Few medical and surgical specialists prescribed gentamicin based on culture results, and in some cases continued it even when culture showed that it was not indicated.
Previous studies have examined the inappropriate use of aminoglycosides;27,28 in one, 10.2% of antibiotic-days were deemed inappropriate.29 An education program for junior medical officers improved appropriate prescribing of gentamicin from 52% to 78%.30
As our study did not include a control group, it is not possible, from our data alone, to establish an unequivocal causal link between gentamicin and vestibulotoxicity. However, published clinical1-8 and experimental31,32 studies remove any doubt.
As there is always a risk of vestibulotoxicity with gentamicin, regardless of dose or serum level, it should be given only as recommended by antibiotic guidelines13 and when there is no safer alternative. Clinicians prescribing gentamicin should use bedside methods to monitor for vestibulotoxicity,10,22 although this is possible only in conscious, cooperative patients. Stopping gentamicin treatment early could prevent further damage, allowing some hair cell regeneration and recovery of vestibular function.33
Our report of 103 cases seen over 23 years suggests that vestibulotoxicity is rare (Addendum). However, our criteria excluded patients in whom we were unable to obtain dosing and clinical details, those with pre-existing renal failure, and those with partial bilateral9 or unilateral vestibulotoxicity.34
1 Definitions of severe bilateral vestibular loss
Caloric testing: < 4°/s peak nystagmus slow-phase velocity from each ear in response to 30°C and 44°C stimulation or < 6°/s in response to 0°C stimulation, or both.
Rotational testing: < 10°/s peak nystagmus slow-phase velocity, or time constant of < 8 s, or both, in response to a constant acceleration stimulus of 20°/s2 for 5 s, or 40°/s2 for 3 s or 100°/s2 for 1 s.
The horizontal and the vertical lines showing total dose (33 mg/kg) and daily dose (3.3 mg/kg/day), respectively, represent a typical dose of 240 mg/day given for 10 days to a 72 kg patient. Note that 50 patients had less than this total dose, 48 patients had less than this daily dose, and 29 patients had both.
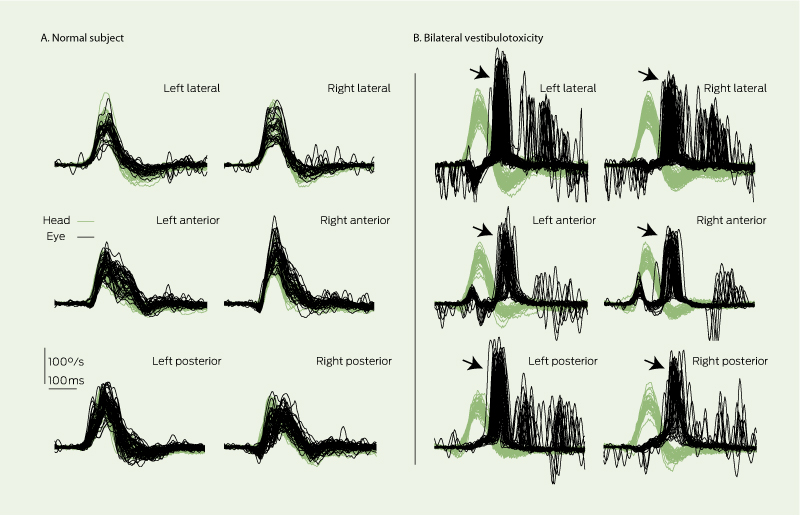
(A) In a normal subject, eye velocity (polarity inverted for ease of comparison) exactly matches head velocity for lateral, anterior and posterior vestibulo-ocular reflexes. (B) In a patient with severe gentamicin vestibulotoxicity, vestibulo-ocular reflexes from all six semicircular canals are severely deficient; patient makes salvos of catch-up saccades (arrows), often visible to the clinician.22 * ICS Video vestibulometry, GN Otometrics, Schaumburg, Illinois, USA, and Tåstrup, Denmark.
We have seen two further patients with severe bilateral gentamicin vestibulotoxicity.
An 87-year-old woman (57 kg and eGFR > 60 mL/min/1.73 m2) was given gentamicin prophylaxis before (240 mg) and after (160 mg) aortic valve replacement surgery in late 2009. Caloric responses are now absent. Moderately severe, bilateral, symmetrical sensorineural hearing loss is unchanged from an audiogram in 2005. She is now severely disabled by imbalance and virtually housebound.
A 60-year-old woman (60 kg and eGFR, > 60 mL/min/1.73 m2) was given gentamicin (400 mg one day and 320 mg the next day) for febrile neutropenia after chemotherapy for breast cancer in early 2012. Her caloric and rotational responses are absent; her hearing is consistent with age. She is moderately disabled, but able to participate in home-based vestibular rehabilitation exercises.
Video 1: Head impulse test in a patient with moderately severe gentamicin vestibulotoxicity
While the patient stares at a distant target, the clinician turns the patient’s head briskly to the left, to the right, up and down. (The head turns need to be fast but small, only about 20° so they do not cause any discomfort.) A subject with a normal vestibulo-ocular reflex is able to keep his eyes fixed on the target by immediately making compensatory smooth eye movements.
This patient cannot do this, most obviously in response to leftward and downward head impulses. Instead, about 150 ms after each head impulse, he makes jerky compensatory eye movements — overt (ie, clinically obvious) “catch-up” saccades — to return his eyes to the target. He has vertical and horizontal impairment of the vestibulo-ocular reflex.
Video vestibulometry reveals that the head impulse test gave positive results in all four directions. The reason the catch-up saccades were not clinically apparent during rightward and upward head rotations was that they were covert (ie, they occurred early and during, rather than after, head rotation).
Video 2: Head impulse test of a patient with severe gentamicin vestibulotoxicity
This patient generates large overt saccades after head rotation to both sides, which are easy for a clinical observer to detect. In slow motion, during head rotation to both sides, the eyes move with the head and jump back to the target with a delayed overt saccade after head rotation. (Reproduced with permission from: Weber KP, Aw ST, Todd MJ, et al. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology 2009; 72: 1417-1424.)
Video 3: Video measurement of a horizontal head impulse test of the patient in Video 2
While the patient stares at a distant target, the clinician (this time from behind) turns the patient’s head briskly to the left and to the right. The patient wears ICS video vestibulometric goggles (GN Otometrics, Schaumburg, Illinois, USA; and Tåstrup, Denmark.) and the clinician watches the results on a screen. These show that the eye velocity, inverted for ease of comparison (yellow traces), is much smaller than the head velocity (blue or red traces) indicating the measured deficit in the horizontal vestibulo-ocular reflex. These data are shown in more detail in Video 4.
Video 4: Horizontal vestibulo-ocular reflex deficit shown in three dimensions
The top two surfaces show the video-vestibulometric results from the patient in Video 2 and Video 3; for comparison, the lower two show results from a subject with a normal vestibulo-ocular reflex. The surfaces are rotating for easy inspection (blue surfaces = head velocity; red surfaces = eye velocity in three dimensions). The surfaces are superimposed eye velocity, which is inverted (ie, polarity reversed) for ease of comparison with head velocity. The lowest initial peaks of each surface represent the lowest stimulus (head) and response (eye) velocities, and the highest peaks are the highest velocities. In the normal subject, the red (eye) and blue (head) velocities are almost identical, indicating a vestibulo-ocular reflex that compensates perfectly. In the patient, the red surfaces are at first much smaller than the blue surfaces, indicating the severe deficit in the vestibulo-ocular reflex at all head velocities; then there is a shower of spikes — the “catch-up” saccades clinically evident in Video 1 and Video 2. (For more information about impulsive testing see www.headimpulse.com)
Video 5: A simulation of impaired dynamic visual acuity due to oscillopsia during vertical head shaking
The simulation shows the visual experience of a patient with severe bilateral vestibular impairment (left), compared with a normal subject (right), during vertical head movement. Note the decreased visual acuity caused by impairment of the vertical vestibulo-ocular reflex in the patient versus preserved visual acuity, with stabilisation of the line-of-sight by normal vestibulo-ocular reflex eye movements. In patients with bilateral vestibular loss, oscillopsia occurs during walking or running.
Video 6: Positive foam Romberg test in a patient with severe bilateral severe gentamicin vestibulotoxicity
The patient has no problem standing with feet together and eyes closed on a firm surface, but is unable to do so on a foam surface. The foam surface disrupts proprioception, the only means of postural stability when neither visual nor vestibular inputs are available to stabilise posture. A control subject is able to stand with eyes closed on a foam surface.
Received 5 July 2011, accepted 5 March 2012
- Rebekah M Ahmed1
- Imelda P Hannigan1
- Hamish G MacDougall2
- Raymond C Chan1
- G Michael Halmagyi1
- 1 Department of Neurology, Royal Prince Alfred Hospital, Sydney, NSW.
- 2 Vestibular Research Laboratory, School of Psychology, University of Sydney, Sydney, NSW.
We thank Dr Richard Benn for helpful discussions over many years and Dr Vicki Levidiotis for reviewing the manuscript. The study was supported by Garnett Passe and Rodney Williams Memorial Foundation (Hamish MacDougall) and National Health and Medical Research Council Grant 245515 (Michael Halmagyi).
Michael Halmagyi and Hamish MacDougall have acted as unpaid consultants for GN Otometrics.
- 1. Dobie RA, Black FO, Pezsnecker SC, Stallings VL. Hearing loss in patients with vestibulotoxic reactions to gentamicin therapy. Arch Otolaryngol Head Neck Surg 2006; 132: 253-257.
- 2. Seemungal BM, Bronstein AM. Aminoglycoside ototoxicity: vestibular function is also vulnerable. BMJ 2007; 335: 952.
- 3. Halmagyi GM, Fattore CM, Curthoys IS, Wade S. Gentamicin vestibulotoxicity. Otolaryngol Head Neck Surg 1994; 111: 571-574.
- 4. Black FO, Pesznecker S, Stallings V. Permanent gentamicin vestibulotoxicity. Otol Neurotol 2004; 25: 559-569.
- 5. Ishiyama G, Ishiyama A, Kerber K, Baloh RW. Gentamicin ototoxicity: clinical features and the effect on the human vestibulo-ocular reflex. Acta Otolaryngol 2006; 126: 1057-1061.
- 6. Ariano RE, Zelenitsky SA, Kassum D. Aminoglycoside-induced vestibular injury: maintaining a sense of balance. Ann Pharmacother 2008; 42: 1282-1289.
- 7. Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA 1998; 279: 541-544.
- 8. Zingler VC, Cnyrim C, Jahn K, et al. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol 2007; 61: 524-532.
- 9. Weber KP, Aw ST, Todd MJ, et al. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology 2009; 72: 1417-1424.
- 10. Vital D, Hegemann SC, Straumann D, et al. A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg 2010; 136: 686-691.
- 11. Vereeck L, Truijen S, Wuyts FL, Van de Heyning PH. The dizziness handicap inventory and its relationship with functional balance performance. Otol Neurotol 2007; 28: 87-93.
- 12. Halmagyi GM, Yavor RA, McGarvie LA. Testing the vestibulo-ocular reflex. Adv Otorhinolaryngol 1997; 53: 132-154.
- 13. Antibiotic Expert Group. Therapeutic guidelines: antibiotic. Version 14. Melbourne: Therapeutic Guidelines Limited, 2010.
- 14. Herdman SJ. Vestibular rehabilitation. 3rd ed. New York: Davis, 2007: 309-359.
- 15. Gates, GA, Cooper JC, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983-1985. Part I. Basic audiometric test results. Ear Hear 1990; 11: 247-256.
- 16. Pearson JD, Morrell CH, Gordon-Salant S, et al. Gender differences in a longitudinal study of age-associated hearing loss. J Acoust Soc Am 1995; 97: 1196-1205.
- 17. Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med 2006; 354: 1856-1857.
- 18. Moore RD, Smith CR, Lietman PS. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis 1984; 149: 23-30.
- 19. Lancaster JL, Mortimore S, McCormick M, Hart CA. Systemic absorption of gentamicin in the management of active mucosal chronic otitis media. Clin Otolaryngol Allied Sci 1999; 24: 435-439.
- 20. Stavroulaki P, Apostolopoulos N, Dinopoulou D, et al. Otoacoustic emissions – an approach for monitoring aminoglycoside induced ototoxicity in children. Int J Pediatr Otorhinolaryngol 1999; 50: 177-184.
- 21. JC. Living without a balancing mechanism. N Engl J Med 1952; 246: 458-460.
- 22. MacDougall HG, Weber KP, McGarvie LA, et al. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 2009; 73: 1134-1141.
- 23. Gentamicin Information Center. About gentamicin poisoning. http://www.gentamicin.com (accessed Mar 2012).
- 24. Appel GB. Aminoglycoside nephrotoxicity. Am J Med 1990; 88: 16S-20S.
- 25. Prins JM, Buller HR, Kuijper EJ, et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993; 341: 335-339.
- 26. Raveh D, Koypt M, Hite Y, et al. Risk factors for nephrotoxicity in elderly patients receiving once-daily aminoglycosides. QJM 2002; 95: 291-297.
- 27. English WP, Williams MD. Should aminoglycoside antibiotics be abandoned? Am J Surg. 2000; 180: 512-515.
- 28. Leong CL, Buising K, Richards M, et al. Providing guidelines and education is not enough: an audit of gentamicin use at The Royal Melbourne Hospital. Intern Med J 2006; 36: 37-42.
- 29. Dunagan WC, Woodward RS, Medoff G, et al. Antibiotic misuse in two clinical situations: positive blood culture and administration of aminoglycosides. Rev Infect Dis 1991; 13: 405-412.
- 30. Johnson MW, Mitch WE, Heller AH, Spector R. The impact of an educational program on gentamicin use in a teaching hospital. Am J Med 1982; 73: 9-14.
- 31. Wu WJ, Sha SH, Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurootol 2002; 7: 171-174.
- 32. Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem 2009; 108: 1226-1236.
- 33. Black FO, Gianna-Poulin C, Pesznecker SC. Recovery from vestibular ototoxicity. Otol Neurotol 2001; 22: 662-671.
- 34. Ahmed RM, MacDougall HG, Halmagyi GM. Unilateral gentamicin vestibulotoxicity. Otol Neurotol 2011; 32: 1158-1162.





Abstract
Objective: To review patients with severe bilateral vestibular loss associated with gentamicin treatment in hospital.
Design and setting: A retrospective case series of presentations to a balance disorders clinic between 1988 and 2010.
Main outcome measures: Relationship between vestibulotoxicity and gentamicin dose or dosing profile; indications for prescribing gentamicin.
Results: 103 patients (age, 18–84 years; mean, 64 years) presented with imbalance, oscillopsia or both, but none had vertigo. Only three noted some hearing impairment after having gentamicin, but audiometric thresholds for all patients were consistent with their age. In all patients, the following tests gave positive results: a bilateral clinical head-impulse test, a vertical head-shaking test for vertical oscillopsia, and a foam Romberg test. In 21 patients, imbalance occurred during gentamicin treatment (ignored or dismissed by prescribers in 20) and in 66 after treatment; the remaining 16 could not recall when symptoms were first noticed, except that it was after gentamicin treatment in hospital. Total gentamicin dose range was 2–318 mg/kg (mean, 52 mg/kg), daily dose range was 1.5–5.6 mg/kg (mean, 3.5 mg/kg), and duration was 1–80 days (mean, 17 days). Six patients had only a single dose; 26 had five or fewer doses. Serum gentamicin levels, measured in 82 patients, were in the recommended range in 59. Time to diagnosis ranged from 4 days to 15 years. Nephrotoxicity developed in 43 patients. Gentamicin dosage complied with contemporary or current Australian antibiotic guidelines in under half the patients.
Conclusions: Gentamicin ototoxicity is vestibular, not cochlear, producing permanent loss of balance, but not of hearing. Gentamicin can be vestibulotoxic in any dose, in any regimen, at any serum level.