Grading system for recommendations in Practical neurology*
Grade C: “Satisfactory or poor” — non-randomised case series and opinions of experts
* Adapted from the National Health and Medical Research Council.
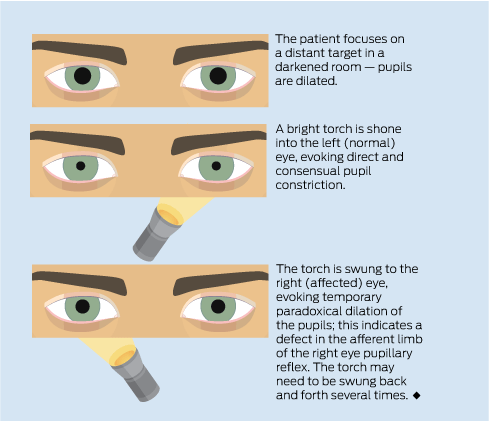
Unilateral visual impairment: Visual impairment in one eye only localises the abnormality to either the eye or the optic nerve. The “swinging flashlight test” detects a difference in the visual stimulus from each eye that reaches the efferent limb of the pupillary reflex when the two eyes are stimulated alternately (Box 1). In this test, paradoxical dilation of the pupil on direct light stimulation indicates a relative afferent pupillary defect (Marcus Gunn pupil) and is pathognomonic of a process that affects the optic nerve, or less commonly the retina, in one eye. Conversely, this test is usually unaffected by conditions that interfere with the transmission of light through the eye itself, such as corneal injury, cataract and processes that make the anterior chamber or the vitreous humour cloudy.
Differential diagnoses of unilateral visual impairment localised clinically to the optic nerve are summarised in Box 2.
Optic neuritis is primarily a clinical diagnosis, and investigations are largely adjunctive. Clinical features that would make a diagnosis of optic neuritis unlikely are listed in Box 3.
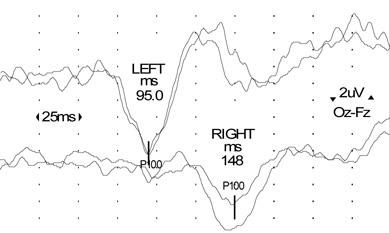
Visual evoked potentials (VEPs): In a typical clinical presentation of optic neuritis, as in this case, recording VEPs is not mandatory, but the presence of a delayed potential with preserved morphology helps confirm the diagnosis of demyelination (Box 4).
Magnetic resonance imaging (MRI): Brain MRI is the most helpful investigation, as it provides aetiological and prognostic information. In patients with a “clinically isolated syndrome” (a single clinical demyelinating event such as optic neuritis) and MRI findings consistent with more widespread demyelination, the risk of a recurrent symptomatic demyelinating episode over the subsequent decade — and hence clinically definite MS — is approximately 80%.1 The diagnosis of MS requires the dissemination of lesions in both space and time. Although a second clinical attack remains the “gold standard” for dissemination in time, new diagnostic criteria (the 2010 revisions to the McDonald criteria,2 which incorporate information obtained from MRI to show dissemination in time and place) permit a diagnosis of MS to be made after a single clinical demyelinating event.
Mary was referred urgently to a neurologist, with a view to urgent MRI. VEPs showed significant prolongation of the cortical potential (P100) latency, indicating slowed conduction in the right optic nerve consistent with a demyelinating process. Results of a full blood count, tests for ESR, fasting glucose level and angiotensin-converting enzyme level, and screening of serum for autoantibodies were unremarkable.
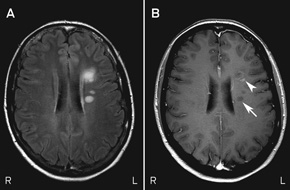
MRI scans of Mary’s brain and orbits showed altered signal intensity within the retrobulbar portion of the right optic nerve. They also showed six small areas of altered signal intensity in the supratentorial white matter affecting the periventricular and subcortical regions, one of which exhibited enhancement with the contrast agent gadolinium (Box 5). The simultaneous presence of enhancing and non-enhancing brain lesions (ie, actively inflamed and older lesions) is sufficient information to prove dissemination in time, and Mary could be told that she has MS without waiting for a second attack.
Therefore, based on the 2010 McDonald criteria for MS, the final diagnosis for Mary was optic neuritis due to MS (Box 6).
Optic neuritis usually resolves spontaneously over weeks to months, and simple analgesia and observation are often sufficient during the acute phase. In severe attacks (visual acuity 6/60 or worse), or when a patient has an occupational or other need to recover vision faster than the natural history of the condition, consideration can be given to the use of high-dose intravenous methylprednisolone (usually administered on an outpatient basis as 1 g/day over 3–5 days), which rapidly reduces pain, limits inflammation and hastens functional recovery (Grade A evidence).4
However, there is no difference in the ultimate visual outcome in patients treated with intravenous steroids versus observation alone,5 thus use of such therapy is controversial. Results of the pivotal Optic Neuritis Treatment Trial suggest that administration of moderate-dose oral prednisone (60 mg/day) to patients with acute optic neuritis increases the risk of recurrence and should therefore be avoided (Grade A evidence).4
The initiation of disease-modifying therapy in patients with a clinically isolated episode of optic neuritis and a diagnosis of MS based on MRI is controversial. There is an increasing body of clinical, neuropathological and MRI evidence to suggest that there may be a window of opportunity during which immunomodulatory therapies, such as interferon β (subcutaneous or intramuscular) and glatiramer acetate (subcutaneous), should be commenced. In part, this has been confirmed by randomised controlled trials in which the initiation of such therapy has been shown to reduce the risk of developing clinically definite MS at 2–3 years by up to 50% (Grade A evidence),6 and reduce disability at 3 years (Grade B evidence),7 compared with delayed treatment. While long-term data favouring the treatment of patients with a clinically isolated syndrome are lacking, there is mounting circumstantial evidence that links inflammation in early MS with chronic axonal loss in the later stages of the disease, supporting the early introduction of immunomodulatory therapy.
The therapeutic options for managing MS have been expanded by the advent of new disease-modifying agents including natalizumab, which is administered as a monthly intravenous infusion, and fingolimod, the first oral agent for the condition.8,9 While considered more efficacious than conventional immunomodulatory treatment, these agents have short- and medium-term risks associated with immunosuppression and systemic effects. Natalizumab therapy can, rarely, be associated with progressive multifocal leukoencephalopathy (PML), a potentially fatal viral infection of the CNS. The risk of PML is determined partly by treatment duration, previous exposure to immunosuppressive therapies and the presence of serum antibodies to the causative agent, JC virus. Fingolimod, a novel immunosuppressive agent, has rare short-term cardiovascular and ophthalmic side effects (bradyarrhythmia and macular oedema, respectively); the risk of potential long-term hazards, such as serious opportunistic infection, is unknown.
Mary’s visual acuity had spontaneously recovered to 6/6 in the affected eye at follow-up 6 weeks after presentation. Minor impairment of right eye colour perception persisted. She subsequently commenced subcutaneous interferon β-1b, 250 μg every 2 days, prescribed by her neurologist and initiated in her home by MS Australia nursing staff. She was advised to use adequate contraception while taking interferon β-1b. Her injection technique was reviewed in the MS clinic 8 weeks later, at which stage she had mild interferon-related flu-like symptoms that were ameliorated by paracetamol. At 6 months, there had been no further discrete neurological attacks, results of a neurological examination were normal and brain MRI showed no new or enhancing (active) lesions. Mary continued to work full time as a sales consultant and had no immediate plans to become pregnant. Six-monthly reviews in the MS clinic were scheduled, with instructions for Mary to contact the MS nurse specialist if she developed any new, sustained neurological symptoms in the interim.
2 Differential diagnoses of unilateral visual impairment localised clinically to the optic nerve
Optic neuritis (immune-mediated inflammation of the optic nerve) can occur in isolation or can be related to multiple sclerosis
Compressive optic neuropathy can be secondary to a tumour (benign or malignant) or secondary to a carotid-ophthalmic aneurysm
Ischaemic optic neuropathy may be arteritic (usually in older patients, and there may be other features of giant cell arteritis) or non-arteritic (more common in patients with hypertension, diabetes mellitus, a history of smoking, and small optic discs with a small cup-to-disc ratio)
Malignant infiltration of the optic nerve (by glioma, leukaemia, lymphoma or carcinoma) merits consideration in cases that are atypical (eg, visual impairment that evolves over weeks to months)
Optic neuropathy of other causes including diabetes (diabetic papillopathy), neuromyelitis optica (NMO or Devic’s disease, a rare inflammatory condition that commonly results in severe visual loss), granulomatous optic neuropathy due to sarcoidosis, systemic autoimmune diseases (eg, Sjögren’s syndrome or systemic lupus erythematosus), and rare infectious diseases (eg, cat-scratch disease, Lyme disease, toxoplasmosis or syphilis)
3 Atypical features of unilateral visual impairment that suggest a diagnosis other than idiopathic optic neuritis
Gradually progressive visual impairment over weeks to months*
Absence of pain on eye movement
Age > 50 years and/or known vascular risks
Severe optic disc swelling*
Bilateral optic disc swelling*
Preserved colour vision
Absence of a relative afferent pupillary defect
Extensive vitreous cellular reaction on slit lamp examination*
6 Pathophysiology of multiple sclerosis (MS)
In relapsing MS, the clinical features correlate with the anatomic localisation of lesions, such as the optic nerve. Most cerebral lesions are clinically silent, but those occurring in “eloquent areas”, such as the optic nerve and spinal cord, are usually symptomatic. The symptoms that accompany optic neuritis and other MS lesions are caused by focal inflammation, impaired axonal conduction due to demyelination, and axonal loss. Recovery, which is often incomplete, is the rule in early relapsing MS and is largely attributable to resolution of inflammation and remyelination. However, there is no discernible regeneration of axons in the CNS, and loss of axons correlates with the development of irreversible disability.3
- Michael H Barnett1,2
- Gurjit Chohan2
- Leo Davies1
- 1 Royal Prince Alfred Hospital, Sydney, NSW.
- 2 Brain and Mind Research Institute, Sydney, NSW.
Michael Barnett has served on scientific advisory boards for Merck Serono, Novartis, and Sanofi-Aventis, and has received research support from Multiple Sclerosis Research Australia and the Nerve Research Foundation (University of Sydney); his institution has received unencumbered research grants from Bayer, Biogen Idec, Novartis and Sanofi-Aventis. Gurjit Chohan is a recipient of a Merck Serono Multiple Sclerosis Fellowship at the Brain and Mind Research Institute.
- 1. Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002; 346: 158-164.
- 2. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292-302.
- 3. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278-285.
- 4. Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1992; 326: 581-588.
- 5. Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology 1994; 101: 1771-1778.
- 6. Coyle PK. Early treatment of multiple sclerosis to prevent neurologic damage. Neurology 2008; 71: S3-S7.
- 7. Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007; 370: 389-397.
- 8. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387-401.
- 9. Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348: 15-23.





Summary
Subacute unilateral visual impairment accompanied by pain on eye movement is characteristic of optic neuritis.
Most cases of optic neuritis resolve spontaneously, and acute treatment with intravenous steroids hastens recovery but does not alter the ultimate visual outcome.
Brain magnetic resonance imaging (MRI) may permit a diagnosis of multiple sclerosis (MS) to be made after a single clinical demyelinating event such as optic neuritis.
Current evidence supports the introduction of disease-modifying therapy in patients with a single clinical event such as optic neuritis and brain MRI compatible with MS.
The diagnosis of MS is a confronting life event associated with significant personal, social and financial burdens. The diagnosing neurologist should provide a detailed explanation of the disease and its clinical spectrum and introduce the patient to the wide range of support services, educational material and MS clinics.