Methamphetamine use is common internationally, and Australia has one of the highest rates of use in the world.1 Users risk a range of negative effects, including dependence,2 issues with personal relationships and employment, and mental health problems.3
Up to a quarter of methamphetamine users report psychiatric symptoms of sufficient severity to warrant hospitalisation.4 In particular, several Australian studies have found high rates of mood disorder among methamphetamine users, with up to 75% reporting symptoms of anxiety or depression.5 Depressive symptoms are even higher among methamphetamine users than among cocaine users, and persist longer.6 It is often comorbid conditions such as depression that prompt users of methamphetamines to seek treatment,7 with general practitioners often being the first port of call.8
The existence of depression prior to treatment has been associated with poorer treatment adherence and poorer methamphetamine use outcomes among methamphetamine users in the United States.9 Despite the high prevalence of depression among methamphetamine users, little is known about the clinical course of depression during treatment for methamphetamine use or how depression affects clinical outcomes.9
To our knowledge, our study is the first to examine the relationship between depression and the response to treatment for methamphetamine use. Our aim was to determine whether depression among regular methamphetamine users moderates the response to brief psychological treatment focused on methamphetamine use. It was expected that participants with comorbid depression would report more severe symptom profiles at baseline and a poorer response to treatment for methamphetamine use.
A detailed description of our study methods has been reported elsewhere.10,11 We give only a brief outline here.
The study was conducted between 2001 and 2005 at two sites in Australia: the Hunter Region of New South Wales and the city of Brisbane, Queensland.
The inclusion criterion was current use of methamphetamine, at least once a week, and participation was limited to people aged 16 years or over. People with psychotic disorder or suicidal ideation were excluded. Potential participants were screened by telephone to determine eligibility for the study.
Of a total of 282 people referred, 68 (24%) were excluded for various reasons: failing to meet the inclusion criterion (27), having a psychotic disorder (16), being at high risk of suicide (7), having an acquired cognitive impairment (4), or declining further participation after referral (14).10 This left a final study sample of 214. Participants were self-referred (57 [27%]) or referred from health services (20 [9%]) or drug and alcohol clinical services (137 [64%]), including community counselling, detoxification programs, needle and syringe programs, pharmacotherapy services (eg, for heroin dependence) and general practices.
Basic demographic information was collected, along with the following specific assessments at baseline, 5 weeks and 6 months.
The BDI-II is a 21-item self-report questionnaire used to screen for the presence of depressive symptoms over the previous 2-week period. The maximum possible score is 63 points. It is commonly used to screen for depressive symptoms among people with substance misuse problems.13 Respondents scoring in the clinical range (≥ 20 points) should be referred for further assessment for major depressive disorder.12
The SCID-IV-RV provides a diagnostic, clinician-rated measure of methamphetamine misuse and dependence based on criteria set out in the Diagnostic and statistical manual of the mental disorders, 4th edition (DSM-IV).15 For the purposes of our study, SCID diagnosis was dichotomised into two separate variables, indicative of misuse/no misuse and dependence/no dependence.
The SDS is a five-item self-report scale used to assess dependence. It is based on criteria for psychological dependence outlined in DSM-IV.15 Scores above 4 for methamphetamine users are indicative of dependence, and the maximum possible score is 25.
The OTI quotient (Q) score is a self-report measure of the quantity and frequency of use of 11 substances, including methamphetamine. The OTI Q score represents the average number of use occasions in the previous month. A score of 1 equates to once daily use, a score of 0.14 to once weekly use.
Based on their OTI scores, participants were given a polydrug use rating for the previous month, obtained by adding together the number of drugs in the previous month with an OTI score greater than zero.17
After providing consent, participants completed the baseline assessment, which took about 45 minutes. Reimbursement of expenses ($20) was provided for each assessment completed (at baseline, 5 weeks and 6 months). After the baseline assessment, participants were randomly allocated to one of three treatment groups, with participants and assessors blind to allocation until that point. The treatment conditions offered were: (a) control (provision of a self-help booklet, A user’s guide to speed [available at http://ndarc.med.unsw.edu.au/NDARCWeb.nsf/page/Resources#speed], but no further counselling) (n = 74); (b) two sessions of counselling (n = 74); or (c) four sessions of counselling (n = 66).
Participants allocated to counselling completed their first treatment session immediately after randomisation. Counselling sessions were typically 45–60 minutes in duration. After the initial treatment session, subsequent sessions occurred weekly for either 1 or 3 weeks, depending on the treatment allocation.
Follow-up was at 5 weeks and 6 months after the initial assessment, undertaken by a researcher blind to treatment allocation.
Data were analysed using PASW Statistics 18 for Windows, release 18.0.0 (SPSS Inc, Chicago, Ill, USA).
The sample selected for analysis consisted of participants who completed the baseline assessment and at least one follow-up assessment (at 5 weeks or 6 months). These were defined as “follow-up completers”. Differences between the depressed and non-depressed groups were examined using one-way analysis of variance (ANOVA) and χ2 analyses.
Linear regression was used to predict change in methamphetamine scores between baseline and 5-week follow-up and baseline and 6-month follow-up assessments. Predictors in these models were sex, whether receiving pharmacotherapy for heroin dependence (yes/no), number of treatment sessions attended, and depression status (depressed/not depressed). We created an interaction variable that accounted for the level of depression and number of treatment sessions attended. This was also entered into each linear regression model as a predictor variable. Logistic regression was used to predict abstinence from methamphetamine (yes/no) at 5 weeks and at 6 months using the same set of predictors.
Changes in depression between baseline and 5 weeks (n = 109) or 6 months (n = 111) were examined only for participants who met criteria for comorbid depression at baseline (the depressed group).
Baseline characteristics of the entire sample have been reported elsewhere.11 The mean age of participants was 30 years (range, 16–55 years). No significant differences existed between treatment groups on a range of demographic and treatment variables and the primary measures at baseline, indicating that randomisation was successful.11
Sixty-three per cent of participants (n = 135) met criteria for methamphetamine use and comorbid depression at baseline. Demographic characteristics of participants who completed at least one of the post-treatment assessments (n = 187) are summarised in Box 1. Of these, 155 were assessed at 5 weeks and 153 at 6 months. A significantly higher proportion of males than females were in the non-depressed group (χ21 = 8.697; P = 0.003). Consequently, sex was included as a covariate in subsequent analyses.
Rates of service use were comparable between depression groups. About 20% of each group (27 in the depressed group and 10 in the non-depressed group) had a history of treatment for another mental health condition, such as psychosis, personality disorder or attention deficit hyperactivity disorder. No significant differences existed between depressed and non-depressed participants in terms of previous treatment for a substance misuse problem (χ21 = 0.964; P = 0.326) or psychiatric hospitalisations (χ21 = 1.470; P = 0.480).
As found previously,11 enrolment in pharmacotherapy for heroin dependence was associated with significantly lower baseline methamphetamine use (mean OTI score for methamphetamine use, 0.86 [heroin pharmacotherapy] v 1.61 [no heroin pharmacotherapy]); F1,186 = 8.086; P = 0.005). Although not statistically significant, higher rates of pharmacotherapy for heroin dependence were observed in the depressed group compared with the non-depressed group (29% v 19%; χ21 = 1.811; P = 0.178). Hence pharmacotherapy-for-heroin status was included as a covariate in subsequent analyses.
We have previously reported that urine samples were collected from 19/109 participants (17%) who completed the 6-month follow-up assessment at the research clinics associated with the trial. All test results were consistent with self-reported use of methamphetamines.11
Compared with the non-depressed group, methamphetamine use (based on OTI Q score) was twice as high in the depressed group at baseline (F2,186 = 10.130; P = 0.002 [Box 1]). There was a non-significant tendency for methamphetamine misuse (based on SCID ratings) to be higher in the depressed group (95% v 86%, χ21 = 3.098; P = 0.078). Similarly, methamphetamine dependence (based on SCID ratings) tended to be higher in the depressed group, but not significantly so (91% v 86%; χ21 = 0.529; P = 0.467). Self-reported dependence, as measured by the SDS, was significantly higher for the depressed group (8.86 [SD, 3.28] v 6.25 [SD, 3.69]; F1,186 = 22.135; P < 0.001).
Comorbid depression did not generally affect usage levels of drugs other than methamphetamines (Box 1). Exceptions were the use of benzodiazepines (F1,186 = 7.039; P = 0.009), tobacco smoking (F1,186 = 5.565; P = 0.019), and polydrug use (F1,186 = 7.754; P = 0.006), all of which were significantly higher in the depressed group.
The depressed group attended significantly more treatment sessions than the non-depressed group (mean, 2.88 v 2.08; F1,82 = 4.650; P = 0.003), but overall treatment completion rates were similar for both groups (78% [depressed group] v 67% [non-depressed group]; χ21 = 4.054; P = 0.132).
One-way ANOVAs showed no significant differences between follow-up completers (n = 187) and non-completers (n = 27) at baseline according to age (F1,213 = 0.001; P = 0.972), level of depression (F1,213 = 0.243; P = 0.623), or methamphetamine use (F1,213 = 2.197; P = 0.140). There was also no significant difference in sex distribution between completers and non-completers (χ21 = 0.048; P = 0.827). A significantly higher proportion of follow-up completers than non-completers had attended all of their allocated treatment sessions (58% v 28%; χ21 = 24.337; P < 0.001).
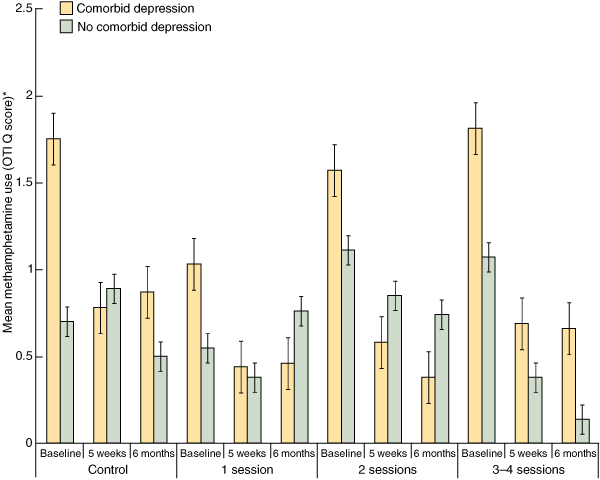
Patterns of methamphetamine use at baseline, 5 weeks and 6 months for participants with and without comorbid depression are summarised in Box 2.
At 5 weeks, changes in methamphetamine use relative to baseline were significantly predicted by the linear regression model containing sex, pharmacotherapy-for-heroin status, number of sessions attended, comorbid depression, and the interaction between sessions attended and presence of comorbid depression (F5,154 = 2.64; P = 0.027; R2 = 0.079). Comorbid depression was the only independently significant predictor of reduction in methamphetamine use at 5 weeks’ follow-up. However, using the same regression model, comorbid depression was not a significant predictor of change in methamphetamine use at 6 months (F5,152 = 1.436; P = 0.215; R2 = 0.043).
Abstinence from methamphetamine use was reported by 18% of participants at 5 weeks and 41% of participants at 6 months. Logistic regression was used to predict abstinence from methamphetamine use at 5 weeks and at 6 months using the following predictors: sex, pharmacotherapy-for-heroin status, number of sessions attended, comorbid depression and the interaction between sessions attended and comorbid depression. Changes relative to baseline were not significantly predicted by the regression model at 5 weeks (χ25 = 4.891; P = 0.429) or at 6 months (χ25 = 8.232; P = 0.144). However, at 6 months, the number of treatment sessions attended was a significant independent predictor of abstinence, suggesting that with each additional session attended (0, 1, 2, 3–4), the likelihood of abstinence increased by 1.5 times.
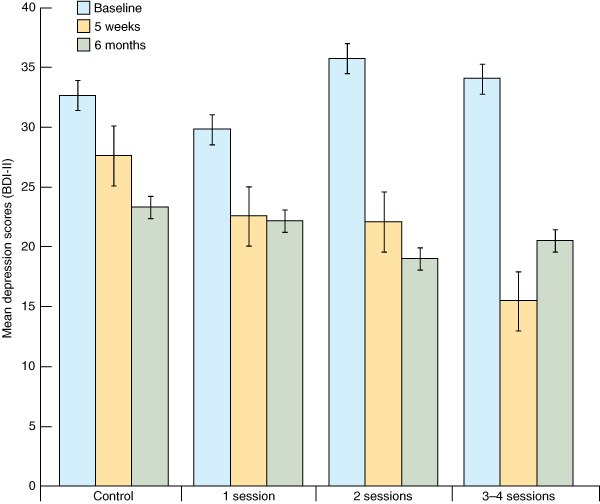
Participants who completed at least one follow-up assessment and who also met criteria for comorbid depression at baseline (n = 135) were selected for closer analysis of their depression scores at follow-up. Mean depression scores reported by this subgroup at each assessment time point are shown in Box 3.
At 5 weeks, changes in depression relative to baseline were significantly predicted by the linear regression equation (F3,108 = 7.192; P < 0.001; R2 = 0.180). The number of treatment sessions attended was the only independent predictor of changes in depression, such that attendance at more treatment sessions was predictive of a greater reduction in depression at 5 weeks. However, at 6 months, the linear regression model was not a significant predictor of change in depression (F3,110 = 1.057; P = 0.370; R2 = 0.031), and there were no independent significant predictors of change.
Most participants who were above the threshold for comorbid depression at baseline remained above that threshold at 5 weeks, regardless of whether they attended one treatment session (67% of group above threshold) or two (53% of group above threshold) or were in the control group (67% of group above threshold). However, only a quarter (n = 16) of those who attended three to four treatment sessions still met criteria for comorbid depression at 5 weeks (χ23 = 12.652, P = 0.005). This apparent treatment effect at 5 weeks among people with comorbid depression at baseline was no longer evident at 6 months, when 48%–51% were above the threshold for comorbid depression, regardless of the number of treatment sessions attended) (χ23 = 0.083; P = 0.994).
Our study is one of the first to report on baseline symptom severity and associated variables relating to methamphetamine users with comorbid depression, and the first to do so with Australian methamphetamine users.
Our results support those of a US study9 in identifying an association between pretreatment depression and increased severity of problems at presentation. Specifically, participants with comorbid depression reported significantly higher levels of methamphetamine use, dependence, polydrug use, tobacco and benzodiazapine use compared with their non-depressed counterparts. It is a significant concern that rates of benzodiazepine use among methamphetamine users with depression (a group more likely to develop dependence) were almost 10 times higher than rates among those without depression. Moreover, benzodiazepine use has been shown to exacerbate depressive symptoms.19,20 It may be that depressed methamphetamine users take benzodiazepines to help them “come down” after methamphetamine use. This potentially keeps them in the cycle of increased use, leading to increased dependence and increased depression. Clinicians are advised to closely monitor and try to minimise benzodiazepine use among depressed methamphetamine users, and perhaps offer alternatives, such as antidepressant medication or psychological treatment, to better manage depression and other undesirable effects of methamphetamine in this group.
Comorbid depression was present in most methamphetamine users in our study, highlighting the need to screen for the presence of depression when treating methamphetamine users. In general, and contrary to expectation, our results showed that the presence of comorbid depression did not have a negative impact on treatment for methamphetamine use, at least in the short term.
Comorbid depression independently predicted greater reduction in methamphetamine use between baseline and 5-week follow-up, but this was not maintained at 6 months. As the depressed group reported significantly higher methamphetamine use at baseline, this reduction may in part be explained by regression to the mean.
There was some evidence for a treatment effect on abstinence from methamphetamine use at 6 months, with attendance at three to four counselling sessions associated with almost five times the chance of achieving abstinence at this time point, independent of depression status. Despite the high initial severity of methamphetamine use and depression, the rates of abstinence achieved by the sample at 6-month follow-up were similar to those reported in other substance use research (eg, 30% abstinence from alcohol21), indicating the potential for methamphetamine users to reach and maintain their treatment goals similarly to users of other drugs.
Several limitations to our study are worthy of note. Firstly, substance use among all participants was heavy and regular, with a range of reported psychological, educational and employment problems present. This likely made it difficult to detect a differential effect of depressive symptoms (present in the majority of cases) on these important outcomes. Nearly three-quarters of participants met criteria for comorbid depression and also had long histories of methamphetamine use. This too may have influenced the ability to effect change using a relatively brief intervention over a short period. We also did not determine whether a person’s depression was independent of methamphetamine use or was methamphetamine-induced, which may have influenced how quickly their depression responded to the treatment provided. In addition, participants were largely recruited from drug and alcohol services, and the results can not necessarily be generalised to other settings.
In summary, our results suggest that it may be important to target comorbid depression in the context of methamphetamine use, given the higher levels of disability and morbidity reported by participants with comorbid depression, and the potential for participants to experience ongoing difficulties that could influence relapse and recovery from both conditions. This seems particularly important given the direct links between depressive symptoms at baseline and subsequent treatment retention and outcomes reported in previous research,22 and the finding that conditions such as depression and substance misuse can respond to psychological and pharmacological treatment.19
1 Baseline demographic profile of methamphetamine users who completed at least one follow-up assessment (n = 187), by presence or absence of comorbid depressive symptoms
|
Depression status at baseline |
||||||||||||||
Demographic factors |
Comorbid depression* (n = 135) |
No comorbid depression (n = 52) |
|||||||||||||
Sex† |
|
|
|||||||||||||
Male |
72 (53%) |
40 (77%) |
|||||||||||||
Female |
63 (47%) |
12 (23%) |
|||||||||||||
Marital status |
|
|
|||||||||||||
Single, never married |
86 (64%) |
36 (70%) |
|||||||||||||
Married/defacto |
31 (23%) |
8 (15%) |
|||||||||||||
Separated/divorced/widowed |
18 (13%) |
8 (15%) |
|||||||||||||
Cultural background |
|
|
|||||||||||||
Aboriginal/Torres Strait Islander |
9 (7%) |
2 (4%) |
|||||||||||||
Anglo-Australian |
115 (85%) |
47 (90%) |
|||||||||||||
European/Pacific rim |
11 (8%) |
3 (6%) |
|||||||||||||
Study site |
|
|
|||||||||||||
Hunter Region |
66 (59%) |
26 (50%) |
|||||||||||||
Brisbane |
69 (51%) |
26 (50%) |
|||||||||||||
Qualifications |
|
|
|||||||||||||
Nil |
27 (20%) |
15 (29%) |
|||||||||||||
Secondary school |
35 (26%) |
14 (27%) |
|||||||||||||
Trade |
59 (44%) |
15 (29%) |
|||||||||||||
Tertiary degree/diploma |
14 (10%) |
8 (15%) |
|||||||||||||
Current income |
|
|
|||||||||||||
Wage/salary |
22 (16%) |
11 (21%) |
|||||||||||||
Pension/benefit |
113 (84%) |
41 (79%) |
|||||||||||||
Drug use scores |
Mean (SEM) |
Mean (SEM) |
|||||||||||||
OTI Q score‡ |
|
|
|||||||||||||
Methamphetamines† |
1.64 (0.14) |
0.87 (0.11) |
|||||||||||||
Alcohol |
2.30 (0.38) |
2.58 (0.67) |
|||||||||||||
Cannabis |
6.53 (1.07) |
6.40 (1.28) |
|||||||||||||
Heroin |
0.13 (0.04) |
0.09 (0.06) |
|||||||||||||
Other opiates |
0.12 (0.04) |
0.03 (0.03) |
|||||||||||||
Cocaine |
0.05 (0.03) |
0.01 (0.01) |
|||||||||||||
Hallucinogens |
0.05 (0.02) |
0.01 (0.01) |
|||||||||||||
Benzodiazepines† |
1.32 (0.27) |
0.14 (0.08) |
|||||||||||||
Tobacco† |
18.46 (1.11) |
13.77 (1.37) |
|||||||||||||
Polydrug use score†§ |
4.53 (0.13) |
3.88 (0.18) |
|||||||||||||
OTI Q = Opiate Treatment Index quotient. * Comorbid depression was defined as Beck Depression Inventory II score ≥ 20. † P < 0.05 for difference between depressed and non-depressed groups. ‡ The OTI Q score indicates quantity/frequency of use during the month prior to assessment. A score of 1 equates to once daily use, a score of 0.14 to once weekly use. § Polydrug use was calculated by summing the number of drugs (including alcohol and tobacco) the participant used in the month prior to assessment. |
|||||||||||||||
2 Self-reported levels of methamphetamine use at baseline, 5 weeks and 6 months, by number of treatment sessions attended and presence of comorbid depression at baseline
 | |||||||||||||||
|
OTI Q = Opiate Treatment Index quotient. * Use occasions per day averaged over previous month. | |||||||||||||||
3 Self-reported levels of depression at baseline, 5 weeks and 6 months among participants with comorbid depression* at baseline (n = 135), by number of treatment sessions attended
 | |||||||||||||||
|
BDI-II = Beck Depression Inventory II. * Comorbid depression was defined as BDI-II score ≥ 20. | |||||||||||||||
- Frances J Kay-Lambkin1,2
- Amanda L Baker2
- Nicole M Lee3
- Linda Jenner3
- Terry J Lewin2
- 1 National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW.
- 2 Centre for Brain and Mental Health Research, University of Newcastle, Newcastle, NSW.
- 3 Turning Point Alcohol and Drug Centre, Melbourne, VIC.
Our research was supported in full by a grant from Australian Department of Health and Ageing. We wish to acknowledge the involvement of the study participants.
None relevant to this article declared (ICMJE disclosure forms completed).
- 1. Australian Institute of Health and Welfare. 2007 National Drug Strategy Household Survey: first results. Canberra: AIHW, 2008. (AIHW Cat. No. PHE 98; Drug Statistics Series No. 20.)
- 2. Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev 2008; 27: 253-262.
- 3. Australian Bureau of Statistics. National Survey of Mental Health and Wellbeing: summary of results, 2007. Canberra: ABS, 2008. (ABS Cat. No. 4326.0.)
- 4. Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict 2004; 13: 181-190.
- 5. Hall W, Farrell M. Comorbidity of mental disorders with substance misuse. Br J Psychiatry 1997; 171: 4-5.
- 6. Rawson RA, Huber A, Brethen P, et al. Status of methamphetamine users 2–5 years after outpatient treatment. J Addict Dis 2002; 21: 107-119.
- 7. Kay-Lambkin FJ, Baker A, Lewin T. The “co-morbidity roundabout”: a framework to guide assessment and intervention strategies and engineer change among people with co-morbid problems. Drug Alcohol Rev 2004; 23: 407-424.
- 8. Scott J, Dickey B. Global burden of depression: the intersection of culture and medicine. Br J Psychiatry 2003; 183: 92-94.
- 9. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al. Identifying methamphetamine users at risk for major depressive disorder: findings from the methamphetamine treatment project at three-year follow-up. Am J Addict 2008; 17: 99-102.
- 10. Baker A, Lee NK, Claire M, et al. Drug use patterns and mental health of regular amphetamine users during a reported “heroin drought”. Addiction 2004; 99: 875-884.
- 11. Baker A, Lee NK, Claire M, et al. Brief cognitive behavioural interventions for regular amphetamine users: a step in the right direction. Addiction 2005; 100: 367-378.
- 12. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, Tex: Psychological Corporation, 1996.
- 13. Dawe S, Loxton N, Hides L, et al. Review of diagnostic and screening instruments for alcohol and other drug use and other psychiatric disorders. 2nd ed. Canberra: Commonwealth of Australia, 2002.
- 14. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR axis I disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute, 2001.
- 15. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text revision. Washington, DC: APA, 2000.
- 16. Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995; 90: 607-614.
- 17. Darke S, Hall W, Wodak A, et al. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. Br J Addict 1992; 87: 733-742.
- 18. Baker A, Kay-Lambkin FJ, Lee NK, et al. A brief cognitive behavioural intervention for regular amphetamine users. Canberra: Department of Health and Ageing, 2003.
- 19. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry 2000; 157 (4 Suppl): 1-45.
- 20. Patten SB, Williams JV, Love EJ. Self-reported depressive symptoms following treatment with corticosteroids and sedative-hypnotics. Int J Psychiatry Med 1996; 26: 15-24.
- 21. Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol 2001; 62: 211-220.
- 22. McNulty J, Kouimtsidis C. Outcomes of treatment interventions in drug abuse. Curr Opin Psychiatry 2001; 14: 201-205.





Abstract
Objective: To determine whether the presence of comorbid depression influences response to psychological treatment for methamphetamine use.
Design: Randomised controlled clinical trial.
Setting and participants: Our study was conducted between 2001 and 2005 at two sites in Australia: the Hunter Region of New South Wales and the city of Brisbane, Queensland. The 214 participants, who were all using methamphetamine at least once a week in the month prior to the study, were self-referred or referred from health services or drug and alcohol clinical services. Participants were divided into two groups based on whether or not they had depressive symptoms at baseline.
Interventions: The control group received only a self-help booklet; the two treatment groups received either two or four counselling sessions involving cognitive behaviour therapy and motivational interviewing techniques to manage methamphetamine use.
Main outcome measures: Changes in methamphetamine use and depression at 5 weeks and 6 months after baseline.
Results: Over 70% of participants met criteria for depression at baseline, and depression was associated with significantly greater severity of methamphetamine use and related issues. Benzodiazepine use was significantly higher among depressed than non-depressed participants. Reductions in methamphetamine use between baseline and 5 weeks were independently predicted by comorbid depression, in favour of increased change among those with baseline depression. Depressed participants who received three or four counselling sessions showed a significant reduction in depression at 5 weeks. However, reductions in methamphetamine use and depression compared with baseline were no longer evident at 6 months.
Conclusions: Over the short term, comorbid depression did not negatively affect response to treatment, with some evidence of a dose–response treatment effect for reduction in depression. This was not maintained at 6 months, indicating that methamphetamine-focused treatment may not enable people with comorbid depression to make sustained improvement at the level of their counterparts without depression.
Trial registration number: ACTRN12611000355976.