A 62-year-old woman with an autoimmune disease presented with panuveitis and was treated with immune suppression. She subsequently developed herpetic acute retinal necrosis and later died of herpes simplex encephalitis. Acute retinal necrosis usually occurs months to years after herpes simplex encephalitis. In our case, the ocular findings were present for 5 weeks before the encephalitis presented. To our knowledge, this is the first Australian case of acute retinal necrosis preceding herpes simplex encephalitis. (MJA 2011; 195: 87-88)
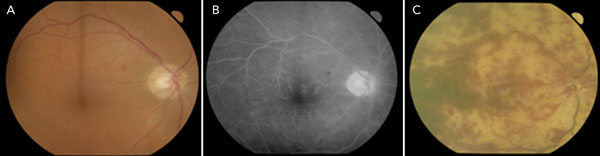
Her initial visual acuity was 6/9 in her right eye and “hand movements” in her left eye. There was a relative afferent pupil defect in her left eye. Her anterior chamber had large mutton-fat keratic precipitates, fibrin and posterior synechiae — all indicative of a granulomatous uveitis. There was no fundal view of the left eye. An ultrasound scan of the left eye showed vitreous debris and a retinal detachment. Fundal examination of the right eye showed one or two small scattered intraretinal haemorrhages (Box 1, A).
Fluorescein angiography (Box 1, B) of the right eye showed mild leakage from the disc and vessels in keeping with an early vasculitis. There was no view of the left fundus.
Two days later, there was a marked deterioration in the patient’s vision. Her visual acuity was recorded as “no perception of light” in both eyes. She had no headache, but she was febrile. Her right eye now had anterior chamber and vitreous cells and widespread retinal haemorrhages (Box 1, C).
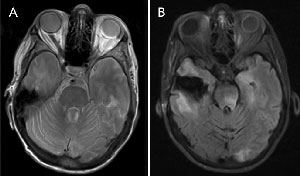
Magnetic resonance imaging (MRI) of the patient’s brain showed an area of encephalomalacia in keeping with her previous surgery. Inflammatory changes and a retinal detachment were noted in the left globe. The optic nerves appeared normal. No orbital masses were noted, and no cavernous sinus pathological features were observed (Box 2, A). A surgical vitreous biopsy was performed on her left eye.
She was commenced on intravenous aciclovir. A repeat MRI scan 8 days after the initial scan showed increased signal on flair involving the thalamus, occipital, temporal lobes and brainstem in keeping with an encephalitis (Box 2, B).
Acute retinal necrosis (ARN) associated with HSE has been well described. Most published reports describe the ocular findings following the encephalitis with a variable time course; the mean interval is 6 months but it can range between 10 days and 20 years.1-6 To our knowledge, there has been only one report published of HSE following ARN.7
When ARN follows HSE, it is assumed that a reactivated latent virus is axonally transmitted from the brain to the retina. A retrospective study published in 2008 examined the causative virus among 52 patients with ARN.8 It found that 14% had a history of previous HSE. There were no cases in which ARN preceded the encephalitis.
In one case report, the authors noted that there have been 20 cases of ARN following HSE published in the past 20 years, and only one case of HSE following 3 weeks after ARN.9
HSE remains a serious illness with significant risk of morbidity and death. A high index of suspicion is required to diagnose HSE. Neurons undergo lysis associated with haemorrhage — a process similar to that seen in the eye with ARN. The exact mechanism of cell death is postulated to be a combination of direct virus-mediated and indirect immune-mediated processes.8,10 Without treatment, the brain undergoes severe inflammation and necrosis.
Provenance: Not commissioned; externally peer reviewed.
- 1. Gain P, Chiquet C, Thuret G, et al. Herpes simplex virus type 1 encephalitis associated with acute retinal necrosis syndrome in an immunocompetent patient. Acta Ophthalmol Scand 2002; 80: 546-549.
- 2. Kamel OR, Galloway GD, Trew DR. Delayed onset acute retinal necrosis 20 years following herpetic encephalitis. Eye (Lond) 2000; 14 Pt 5: 788-789.
- 3. Levinson RD, Reidy R, Chiu MT. Acute retinal necrosis after neonatal herpes encephalitis. Br J Ophthalmol 1999; 83: 123-124.
- 4. Maertzdorf J, Van der Lelij A, Baarsma GS, et al. Herpes simplex virus type 1 (HSV-1)-induced retinitis following herpes simplex encephalitis: indications for brain-to-eye transmission of HSV-1. Ann Neurol 2000; 48: 936-939.
- 5. Pavésio CE, Conrad DK, McCluskey PJ, et al. Delayed acute retinal necrosis after herpetic encephalitis. Br J Ophthalmol 1997; 81: 415-416.
- 6. Preiser W, Doerr HW, Buxbaum S, et al. Acute retinal necrosis six years after herpes simplex encephalitis: an elusive immune deficit suggested by insufficient test sensitivity. J Med Virol 2004; 73: 250-255.
- 7. Pepose JS, Kreiger AE, Tomiyasu U, et al. Immunocytologic localization of herpes simplex type 1 viral antigens in herpetic retinitis and encephalitis in an adult. Ophthalmology 1985; 92: 160-166.
- 8. Vandercam T, Hintzen RQ, de Boer JH, Van der Lelij A. Herpetic encephalitis is a risk factor for acute retinal necrosis. Neurology 2008; 71: 1268-1274.
- 9. Bristow EA, Cottrell DG, Pandit RJ. Bilateral acute retinal necrosis syndrome following herpes simplex type 1 encephalitis. Eye (Lond) 2006; 20: 1327-1330.
- 10. Atherton SS. Acute retinal necrosis: insights into pathogenesis from the mouse model. Herpes 2001; 8: 69-73.





None relevant to this article declared (ICMJE disclosure forms completed).