Lower urinary tract symptoms (LUTS) are common among Australian men, with prevalence of one or more symptoms increasing from less than 20% among men aged under 45 years to 48% of men aged 65–79 years1 and 70% of men aged 80 years and over.2 Not all men with LUTS have benign prostatic hyperplasia (BPH), and not all men with BPH have LUTS.3 Additionally, symptoms caused by BPH can be difficult to distinguish from those resulting from an overactive bladder (OAB).
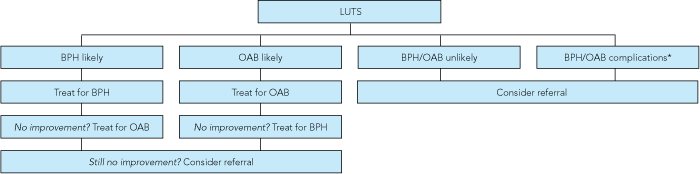
Most men with LUTS will have either BPH or OAB, or both.4 As BPH can be difficult to distinguish from OAB, it may be more practical to determine whether (a) either BPH or OAB is likely or (b) neither are probable, as patients with BPH and OAB can often be managed in the primary care setting, whereas those with uncertain or other diagnoses may require referral to a urologist.
OAB is characterised by urinary frequency, urgency and nocturia,5 whereas patients with BPH may present with any combination of voiding, storage (usually most bothersome) or post-micturition symptoms (Box 1).6
The International Prostate Symptom Score (IPSS) (Box 2) is a validated tool that is used to help determine need for therapy and monitor treatment response. The IPSS ranks symptoms as mild (IPSS 0–7), moderate (8–18) or severe (19–35), and impact on quality of life is rated from 0 (best) to 6 (worst). Although symptom scoring systems are underused in general practice,7 use of the IPSS is encouraged. The IPSS is not a reliable diagnostic tool for LUTS, but serves as a measure of LUTS after the diagnosis is established.
Box 3 summarises the investigations recommended for men who present with LUTS. Prostate-specific antigen (PSA) testing should only be considered to support differential diagnosis (to exclude advanced prostate cancer among older men with symptoms of bladder overflow obstruction), treatment decisions and monitoring (when managed with watchful waiting or 5-α-reductase inhibitors [5ARIs]).
Although most patients with LUTS due to BPH remain clinically stable in the short-to-medium term,8 BPH is a progressive condition and, over time, patients are at increasing risk of symptom deterioration, acute urinary retention and the need for surgical intervention.9 Risk factors for symptom progression should be considered, as they influence treatment choice (Box 4).
The initial approach to a man presenting with LUTS should be to determine whether BPH, OAB or neither is likely (Box 5). Patients with provisional diagnoses other than BPH or OAB should be considered for referral, as should those with disease complications. Patients with a provisional diagnosis of BPH can be treated initially for BPH; if symptoms do not improve, a trial of OAB therapy should be considered. A corresponding approach is applied to patients with a provisional diagnosis of OAB. We recommend that patients whose conditions fail to respond to both BPH and OAB therapy are referred to a urologist. Other indications for referral are summarised in Box 6.
There are four types of treatment available for BPH — watchful waiting, pharmacotherapy, minimally invasive surgical therapies (MISTs) and surgery. Selection depends on disease severity, impact on quality of life, patient preference, presence of complications and fitness for surgery.11
Watchful waiting is the monitoring of a patient without medical or surgical intervention; it generally entails education, reassurance, periodic review and lifestyle advice.8 Its rationale is that BPH, left untreated, does not clinically progress in most men with LUTS (about 85% have stable disease 4 years after diagnosis) and few develop urinary retention or other complications.9 Watchful waiting is generally recommended for men with mild-to-moderate symptoms whose quality of life is not impaired and who have no disease complications.
There are two classes of medications for BPH — α-blockers and 5ARIs.11 α-Blockers can provide prompt symptom relief but do not prevent disease progression, whereas 5ARIs slow disease progression but may take up to 6 months to alleviate symptoms. Treatment choice depends primarily on prostate size (as estimated by DRE and/or PSA level), impact on quality of life, likelihood of progression and affordability (Box 7).
α-Blockers are the main pharmacological treatment for LUTS due to BPH. They act by blocking α1-adrenoceptors in the prostate, with consequent reduction of smooth muscle tone.13 There are three known subtypes of α1-receptors — α1A is predominately expressed in the prostate, α1B in vascular tissue and α1D in the bladder.13 Of the four available α1-blockers, tamsulosin demonstrates selective affinity for α1A and α1D receptors; alfuzosin, prazosin and terazosin show equal affinity for all α1-receptor subtypes.13 Alfuzosin has selective tissue distribution to the prostate. Prazosin has a less favourable side-effect profile than the other medications and requires multiple daily dosing; it is consequently not recommended by overseas BPH guidelines.8,14-16
A comprehensive review of studies comparing α1-blockers concluded that alfuzosin, tamsulosin and terazosin have comparable efficacy with regard to mean improvement in symptom score (30%–45%) and maximal urinary flow rate (15%–30%).13 Alfuzosin and tamsulosin are considered to be better tolerated than terazosin, with fewer cardiovascular adverse effects (dizziness and orthostatic hypotension) and lower rates of treatment discontinuation.13 Other common adverse effects of α-blockers include headaches, asthenia, drowsiness and nasal congestion, although prevalence is similar to placebo.8
With regard to sexual function, abnormal ejaculation is mainly associated with tamsulosin use (incidence, 4%–5%)13 but not with alfuzosin.
Patients taking α-blockers should be reviewed 1 month and 6 months after initiation of therapy. Prazosin and terazosin require dose titration, whereas alfuzosin and tamsulosin are commenced at full therapeutic dose.17 Postural blood pressure changes should be monitored upon initiation and dose titration, with caution exercised in patients using concurrent hypotensive therapy. For the one-third of men who do not experience significant symptom reduction with α1-blockers, treatment should be ceased after 1 month.8
5ARIs are recommended for use by men with large prostates (> 30 mL)18 or PSA levels ≥ 1.4 ng/mL.19 By preventing conversion of testosterone to dihydrotestosterone, they reduce prostatic volume by 18% to 30% and result in decreased risk of clinical progression, acute urinary retention and surgical intervention.19 5ARIs are less effective than α1-blockers at improving IPSS score (15% v 30%–45%, respectively) and urinary flow rate. Symptomatic benefit can take over 3–6 months of treatment. Accordingly, patients with large prostates and bothersome symptoms requiring prompt relief may benefit from combined therapy.
Dutasteride and finasteride are available in Australia.20 Both are similarly efficacious in terms of symptom score and urinary flow rate improvement, with comparable tolerability. Adverse events associated with finasteride include decreased libido (6.4%), impotence (8.1%), decreased ejaculate (3.7%) and uncommonly (< 1%) rash, breast enlargement and breast tenderness.8 Long-term treatment with finasteride reduces PSA; PSA should be multiplied by 2.0 after 1–2 years of treatment, by 2.3 after 2–7 years of treatment and by 2.5 thereafter to allow correct interpretation.21 Patients taking 5ARIs are commonly reviewed 3 and 6 months after initiation.
A double-blind, randomised, parallel-group trial of 4844 men with moderate-to-severe symptoms of BPH demonstrated that the combined use of an α1-blocker (tamsulosin) and a 5ARI (dutasteride) conferred significant improvement in symptom score and quality of life than either drug alone (P < 0.001).20 Significant improvements observed from 3 months were maintained for the 4-year follow-up. Recently, fixed-dose combination pills containing tamsulosin 0.4 mg and dutasteride 0.5 mg have become available at a lower cost than the combined cost of the two single agents.
These results are consistent with those of another trial that showed combination therapy conferred greater clinical benefit than single drug treatment (P < 0.001).9 Accordingly, men with enlarged prostates should be offered combination therapy after balancing costs and side-effect risks.
Saw palmetto, African plum, South African star grass, stinging nettle, and rye pollen are popular herbal remedies for LUTS in Australia. Saw palmetto is the most extensively studied; however, a Cochrane review concluded that it confers “little or no efficacy” in the treatment of BPH symptoms.22
As morbidity from TURP is common, laser surgery is gaining significant popularity. MISTs generally have lower morbidity, but are less effective and are characterised by a higher reoperation rate than more invasive procedures.23 TUIP has symptomatic improvement equivalent to TURP in smaller prostates (≤ 30 mL), but higher rates of subsequent surgery.8 Open prostatectomy is principally considered for very large prostates. Prostatic stents are advocated only in high-risk patients due to common associated morbidity.23
Patients should be advised that these procedures all commonly result in transient storage LUTS. Common early complications following TURP include postoperative bleeding and urinary tract infection. Early urgency incontinence is common (20%–30%) but is unlikely to persist (< 0.5%), and permanent ejaculatory dysfunction occurs in 53%–75% of patients.24
All patients with suspected OAB should be educated regarding bladder training.25 A common approach is as follows:
Gradually increase the time between toilet stops, aiming to reduce the number of voids per day to normal (4–6 voids/day and 1–2 voids at night).
Upon feeling the urge to urinate, delay urination for 1 minute. Gradually increase the delay to 5 minutes. If the urge passes, don’t urinate until the urge returns.
Refrain from pre-emptive voids.
The abnormal and involuntary contractions of the bladder’s detrusor muscle result from stimulation of muscarinic receptors; muscarinic receptor antagonists are accordingly the mainstay of pharmacotherapy. Tricyclic antidepressants (particularly imipramine) and α-blockers can be considered for patients with contraindications to antimuscarinic agents.5
Five antimuscarinics are approved for OAB treatment in Australia: oxybutynin, tolterodine, propantheline (rarely prescribed), solifenacin and darifenacin.17 Oxybutynin is a relatively non-selective antimuscarinic with many years of clinical use; tolterodine is a newer agent and has a similar efficacy and adverse-effect profile. Solifenacin (a competitive, selective M1- and M3-receptor antagonist) and darifenacin (a selective M3-receptor antagonist) are being increasingly used due to their superior adverse effect profiles. The adverse effects of and contraindications to antimuscarinic agents are summarised in Box 8.
The approach to management of LUTS among men has changed significantly over the past decade. Much of this has been on the basis of improved understanding of the natural history of LUTS and clinical trials of newer therapeutic options. This process continues to evolve, and it is anticipated that further understanding of individualised risk for BPH progression will see a more tailored approach with the greater use of different combinations of drug therapy in the future. There are currently several newer pharmacological and MIST options at advanced stages of clinical trials, and it is anticipated that a significant additional number of treatment options will become available over the next 5 to 10 years. Resources for doctors and patients are shown in Box 9.
2 The International Prostate Symptom Score (IPSS)
3 Investigations recommended for men presenting with lower urinary tract symptoms
Urinalysis and urine microscopy
Blood glucose level to screen for diabetes mellitus
Creatinine level to exclude renal impairment
Prostate-specific antigen level to support differential diagnosis (ie, to exclude advanced prostate cancer among older men with bladder overflow obstruction) and treatment decisions, and to monitor response to therapy (watchful waiting or 5-α-reductase inhibitor use)
Involves the recording of date, time of day and night, volume voided and fluid intake over at least 3 days
Helps exclude polyuria, which may be misinterpreted as increased frequency, and conditions associated with nocturnal diuresis (eg, heart failure)
Postvoid residual (PVR) ultrasound
PVR volume > 50 mL has been associated with a higher risk of disease progression in controlled clinical trials; however, PVR may be influenced by voided volume and test conditions. For practical purposes, urology referral should be considered for patients with PVR > 250 mL
4 Risk factors for progression of benign prostatic hyperplasia
Age > 70 years10
Increased prostate-specific antigen level (≥ 1.5 ng/mL)10
Enlarged prostate (> 30 mL)9
Severity of lower urinary tract symptoms or worsening of symptoms over time9
Poor urinary flow rate9
Postvoid residual volume > 50 mL
Failure to respond to medical therapy
Previous episode of acute urinary retention
6 Indications for referral to a urologist
Conditions other than benign prostatic hyperplasia and overactive bladder, including:
Suspected prostate cancer
Meatal stenosis
Potential neurological cause
Complications of benign prostatic hyperplasia:
Urinary retention
Urinary tract infection
Haematuria or benign prostatic bleeding
Uncertain diagnosis
Symptoms impair quality of life
Poor response to pharmacotherapy
Prior genitourinary surgery or trauma
Postvoid residual urine volume > 250 mL
7 Pharmacotherapy for benign prostatic hyperplasia
8 Prescribing antimuscarinic agents
9 Resources for doctors and patients
Andrology Australia (http://www.andrologyaustralia.org) provides information on prostate health, including LUTS, PSA testing, BPH and prostate cancer.
The Continence Foundation of Australia (http://www.continence. org.au) provides information and courses about prostate disorders and urinary incontinence, and distributes maps of public toilet locations.
FingerTip Urology (http://www.bjui.org/FingertipUrology.aspx) provides illustrated slides on “Lower urinary tract symptoms in middle-aged and elderly men”.
Provenance: Not commissioned; externally peer reviewed.
- Henry H Woo1,2
- Michael P Gillman3
- Robert Gardiner4
- Villis Marshall5
- William J Lynch6
- 1 Sydney Urological Associates, Sydney Adventist Hospital, Sydney, NSW.
- 2 Sydney Medical School, University of Sydney, Sydney, NSW.
- 3 Shore Street West Medical Centre, Brisbane, QLD.
- 4 University of Queensland, Brisbane, QLD.
- 5 Faculty of Health Sciences, University of Adelaide, Adelaide, SA.
- 6 Department of Urology, St George Medical Centre, Sydney, NSW.
We thank Scius Solutions for assistance in the preparation of this manuscript. This contribution was funded by an unrestricted educational grant from Sanofi-Aventis.
Henry Woo has received honoraria from Sanofi-Aventis, is a board member for Sanofi-Aventis (Xatral), Ipsen (Diphereline), Hospira (Eligard) and GlaxoSmithKline (Avodart), and has received consultancy fees from American Medical Systems (Greenlight Laser), Janssen-Cilag (Abiraterone), Medivation (MDV3100) and Neotract (Urolift); he also owns stocks in Urolift. He has received payment from AstraZeneca for being a conference organiser, and from Sanofi-Aventis for a conference presentation. He has received payment from GlaxoSmithKline, CSL and Australian Doctor for development of educational presentations.
Michael Gillman has received honoraria from Sanofi-Aventis and is a board member for Sanofi-Aventis (Xatral). Robert Gardiner has received honoraria and support to travel to meetings from Sanofi-Aventis. Villis Marshall has received honoraria from Sanofi-Aventis. William Lynch has received honoraria from Sanofi-Aventis and is a board member for Sanofi- Aventis (Xatral).
- 1. Pinnock C, Marshall VR. Troublesome lower urinary tract symptoms in the community: a prevalence study. Med J Aust 1997; 167: 72-75. <MJA full text>
- 2. Parsons JK, Bergstrom J, Silberstein J, Barrett-Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology 2008; 72: 318-321.
- 3. Kaplan SA. Current role of alpha-blockers in the treatment of benign prostatic hyperplasia. BJU Int 2008; 102 Suppl 2: 3-7.
- 4. Hyman MJ, Groutz A, Blaivas JG. Detrusor instability in men: correlation of lower urinary tract symptoms with urodynamic findings. J Urol 2001; 166: 550-552.
- 5. Ouslander JG. Management of overactive bladder. N Engl J Med 2004; 350: 786-799.
- 6. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002; 187: 116-126.
- 7. Seftel AD, Rosen RC, Rosenberg MT, Sadovsky R. Benign prostatic hyperplasia evaluation, treatment and association with sexual dysfunction: practice patterns according to physician specialty. Int J Clin Pract 2008; 62: 614-622.
- 8. de la Rosette JJ, Alivizatos G, Madersbacher S, et al. EAU guidelines on benign prostatic hyperplasia (BPH). Eur Urol 2001; 40: 256-263.
- 9. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349: 2387-2398.
- 10. Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162: 1301-1306.
- 11. Beduschi MC, Beduschi R, Oesterling JE. Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1A-adrenergic antagonist. Urology 1998; 51: 861-872.
- 12. eMIMS. Sydney: UBM Medica, 2011 (updated May 2011). Subscription only. http://www.mimsonline.com.au (accessed May 2011).
- 13. Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95 Suppl 4: 29-36.
- 14. McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185: 1793-1803.
- 15. National Clinical Guideline Centre. The management of lower urinary tract symptoms in men: pre-publication check. London: Royal College of Physicians, 2010. http://www.nice.org.uk/guidance/index.jsp?action=folder&o=47261 (accessed Apr 2011)
- 16. Nickel JC, Herschorn S, Corcos J, et al. Canadian guidelines for the management of benign prostatic hyperplasia. Can J Urol 2005; 12: 2677-2683.
- 17. Genitourinary drugs. In: Australian medicines handbook. Adelaide: AMH, 2010: 509-520.
- 18. Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomized clinical trials. Urology 1996; 48: 398-405.
- 19. Bruskewitz RC. Quality of life and sexual function in patients with benign prostatic hyperplasia. Rev Urol 2003; 5: 72-80.
- 20. Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57: 123-131.
- 21. Etzioni RD, Howlader N, Shaw PA, et al. Long-term effects of finasteride on prostate specific antigen levels: results from the prostate cancer prevention trial. J Urol 2005; 174: 877-881.
- 22. Tacklind J, MacDonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2009; (2): CD001423.
- 23. Donnell RF. Minimally invasive therapy of lower urinary tract symptoms. Urol Clin North Am 2009; 36: 497-509, vi-vii.
- 24. Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP) — incidence, management, and prevention. Eur Urol 2006; 50: 969-979.
- 25. Continence Foundation of Australia. Bladder training. 2011. http://www.continence.org.au/pages/bladder-training.html (accessed Apr 2011).





Abstract
Lower urinary tract symptoms (LUTS) are common among Australian men over the age of 45 years; most men with LUTS will have benign prostatic hyperplasia (BPH), overactive bladder (OAB), or both.
The cause of LUTS should be diagnosed by assessing symptom severity and excluding of medical or pharmaceutical causes. All men with LUTS should undergo digital rectal examination; other diagnostic tools include urine and blood testing, voiding charts and imaging.
Depending on disease severity, impact on quality of life, patient preference, presence of complications and fitness for surgery, BPH is managed with watchful waiting, pharmacotherapy (α-blockers or 5-α-reductase inhibitors), minimally invasive surgical therapies or surgery.
OAB is initially treated with behavioural therapy; if this is ineffective, pharmacotherapy (usually antimuscarinics) can be used.
Patients with LUTS with a provisional diagnosis other than BPH or OAB, or with complications or poor response to pharmacotherapy, should be referred to a urologist.