We present the first human case of anisakidosis acquired from eating locally caught fish in Australia. A 41-year-old woman experienced gastrointestinal pain, vomiting and diarrhoea of increasing severity over 3 weeks. All symptoms resolved spontaneously after a worm was passed in her faeces. Microscopic examination showed that it was a Contracaecum species larva of the family Anisakidae. Anisakidosis should be considered in patients with gastrointestinal symptoms who have recently eaten seafood. (MJA 2011; 194: 199-200)
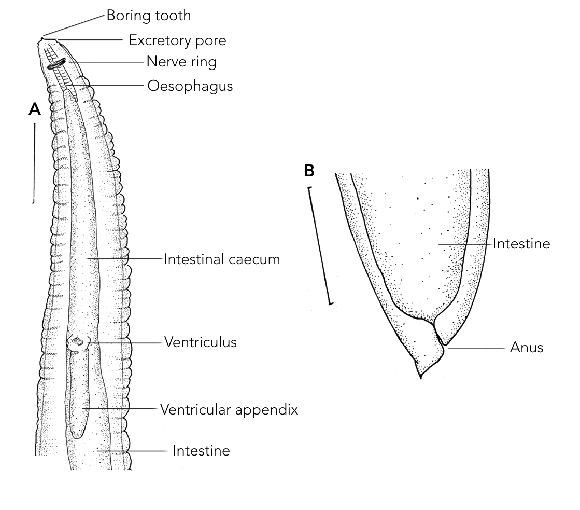
The initial presumptive identification of the larva was an intestinal nematode, possibly a species of the Trichostrongylus or Ascaris genera. However, on further detailed microscopic examination, the larva was identified as a species of Contracaecum (Nematoda: Anisakidae) based on the presence of an intestinal caecum and ventricular appendix (Box) and the position of the excretory pore being at the base of the mouthparts.
Anisakidosis in humans is a well known disease resulting from accidental infestation with larvae of certain genera of anisakids, causing severe gastrointestinal disorders, allergic reaction and even death.1 This is the first reported case of anisakidosis in Australia.
The allergic response can occur against live anisakids or food in which worms were killed by cooking or pasteurisation.2 The pathological changes that occur within the gastrointestinal tract during infestation with anisakids are the combined result of the direct action of the larva during tissue invasion and the complex interaction between the host immune system and the substances released by, or contained within, the parasite.2
In our case, the patient’s symptoms lasted about 3 weeks until a larva was passed in a bowel motion. Several cases of transient luminal anisakidosis have been reported in humans where a larva has been passed hours to weeks after consumption of infested seafood.3 In our case, early symptoms, including vomiting, diarrhoea and abdominal pain, could have been due to unsuccessful attempts of the parasite to penetrate the gastrointestinal wall. The medication prescribed before admission to hospital most likely relieved the symptoms for a limited time. We postulate that the larva survived and moved slowly down the gastrointestinal tract, causing additional symptoms. The sore throat, rhinorrhoea, nasal congestion and cough could have been a concurrent respiratory tract infection or a late hypersensitivity response. Other symptoms could have been due to dehydration as a result of vomiting and diarrhoea.
Infestation with Contracaecum larvae has been reported less frequently than infestation with Anisakis larvae.4,5 In addition, reports of anisakid larvae in faeces are scarce.3 However, over 90% of cases described worldwide were caused by a single larva.2,3 The symptoms reported are diverse and cannot be related to specific morphotype of the parasite. For example, anisakidosis due to Pseudoterranova larvae is mostly benign in the United States, but can be a severe infestation in Japan.6 It is known that Anisakis type I larvae in Japanese mackerels caught on the eastern coast of Japan are less pathogenic than those caught on the western coast of the country.7
Of the many records of anisakidosis worldwide, only a few reports identified the larva to species level. This is due to the lack of species-specific morphological features in larval stages. Recently, molecular approaches have been developed to identify larvae specifically.8 In our case, it was not possible to identify the larva to a species level due to inappropriate preservation of the specimen.
As human infestations occur after eating infested seafood, it is implied that the mackerel eaten by the patient was infested. In Australia, larval and adult stages of various species of anisakids infest a broad variety of fish, including mackerels.8-11 However, our case report represents the first human anisakidosis acquired from eating locally caught fish. Given that the popularity of consuming raw or undercooked fish (eg, sushi) is increasing, it is possible that anisakidosis is underdiagnosed in Australia due to the vague symptoms and limited diagnostic tests. For example, in a clinicopathological study of 92 cases of anisakidosis in Japan, over 60% of cases were diagnosed preoperatively as appendicitis, acute abdomen, gastric tumour or cancer, ileitis, cholecystitis, diverticulitis, tuberculous peritonitis, and cancer of the pancreas.12
- 1. Kliks MM, Fontaine, RE. Human anisakiasis: an update. JAMA 1986; 255: 2605.
- 2. Audicana MT, Kennedy, MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 2008; 21: 360-379.
- 3. Smith JW. Ascaridoid nematodes and pathology of the alimentary tract and its associated organs in vertebrates, including man: a literature review. Helminthol Abstr 1999; 68: 49-96.
- 4. Im K, Shin H, Kim B, et al. Gastric anisakiasis cases in Cheju-do, Korea Republic. Korean J Parasitol 1995; 33: 179-186.
- 5. Yagi K, Nagasawa, K, Ishikura H, et al. Female worm Hysterothylacium aduncum excreted from human: a case report. Jpn J Parasitol 1996; 45: 12-23.
- 6. Desowitz RS. Human and experimental anisakiasis in the United States. Hokkaido Igaku Zasshi 1986; 61: 358-371.
- 7. Suzuki J, Murata R, Hosaka M, et al. Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int J Food Microbiol 2010; 137: 88-93.
- 8. Shamsi S, Gasser R, Beveridge I. Mutation scanning-coupled sequencing of nuclear ribosomal DNA spacers as a tool for the specific identification of different Contracaecum (Nematoda: Anisakidae) larval types. Mol Cell Probes 2010; Oct 8. [Epub ahead of print]. DOI:10.1016/j.mcp.2010.09.003
- 9. Shamsi S, Gasser R, Beveridge I, et al. Contracaecum pyripapillatum n. sp. and a description of C multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitol Res 2008; 103: 1031-1039.
- 10. Shamsi S, Norman R, Gasser R, et al. Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitol Res 2009; 105: 529-538.
- 11. Shamsi S, Norman R, Gasser R, et al. Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol Res 2009; 104: 1507-1525.
- 12. Sakanari JA, McKerrow JH. Anisakiasis. Clin Microbiol Rev 1989; 2: 278-284.





None identified.