Congenital cytomegalovirus (cCMV) infection is the most common serious viral intrauterine infection.1 However, neither pregnant women nor neonates are routinely screened for this in most countries, including Australia. The reported annual incidence in Australia is 3.85 per 100 000,2 which we consider, based on prospective studies, to be a significant underestimate, which is partly due to unreported cases that are asymptomatic at birth or present with isolated sensorineural hearing loss. The prevalence of cytomegalovirus (CMV) at birth, worldwide, is about 0.64%,1 with 10%–15% of congenitally infected infants being symptomatic at birth. In symptomatic infants, mortality is as high as 30% and late-onset sequelae occur in 35%–100%.3 In infants who are asymptomatic at birth, neurodevelopmental sequelae occur in 13.5%, with hearing loss occurring in 12%.3-5
We report an Australian case series which constitutes the longest active nationwide surveillance of cCMV published to date. Cases were reported through a nationwide survey between January 1999 and February 2009, conducted by the Australian Paediatric Surveillance Unit (APSU) (http://www.apsu.org.au). Since initial results for 1999–2003 were reported,6 newborn hearing screening has been introduced in most Australian states (Box 1), and therapeutic studies have been published.7 New diagnostic techniques for cCMV infection allow for retrospective diagnosis of cCMV infection.8,9 For this reason, we compare data from two 5-year periods: 1999–2003 and 2004–2009. We also report a case review of a subset of children, reviewed at two tertiary paediatric institutions up to 108 months of age. Finally, we compare presenting symptoms identified in infants within the first 60 days of life (that is, within the usual period for routine postnatal check-up by a family doctor for Australian infants with particular reference to neurodevelopmental and hearing outcome) with those identified in infants aged over 60 days.
A definite case of cCMV (as previously described6) was defined as a child in whom CMV was identified in the first 3 weeks of life, from urine, blood, saliva or any tissue taken by biopsy.
A suspected case was defined as any child up to 12 months of age, from whom CMV was isolated, or who was positive for CMV IgM and in whom clinical features were suggestive of intrauterine CMV infection.6
Cytomegalovirus was detected using cell culture, shell vial assays (direct immunofluorescence identification of immediate early CMV antigen) or by histopathological examination of tissue specimens.6 Serum IgM antibody to CMV was determined using an IgM capture enzyme immunoassay (DiaSorin, Saluggia, Italy). Additional infants with cCMV were identified as a result of another study on the seroprevalence of CMV among pregnant women and women identified with documented antenatally acquired CMV infection.10
The extraction of CMV DNA from newborn screening cards was performed as previously reported11 with few modifications.8 Three disks of 3 mm diameter were prepared from newborn screening cards using a Wallac DBS Puncher (product number 1296-071) (PerkinElmer, Turku, Finland). Blood was eluted from the newborn screening cards by incubating in 45 mL of minimum essential media at 55°C for 60 minutes, followed by heating at 100°C for 7 minutes. Samples were rapidly cooled, centrifuged at 10 000 g for 3 minutes and frozen at − 80°C for at least 1 hour before further testing.
For extraction of CMV DNA from urine samples, a MagNA Pure semi-automated nucleic acid extraction machine (Roche Applied Science, Mannheim, Germany) was used. The samples were stored at − 20°C before polymerase chain reaction (PCR) testing for the presence of the CMV glycoprotein gene gp58, and for the major immediate gene.8,12
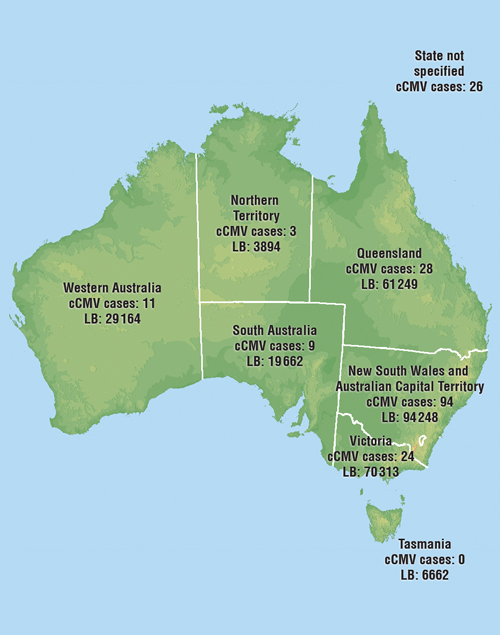
There were 363 notifications of cCMV to the APSU from January 1999 to February 2009, and 237 (65%) follow-up questionnaires were returned by doctors. Of these, 195 were classified as definite or probable cCMV, using the case definition. The notifications were from across Australia, with a majority from New South Wales, which has about one-third of all Australian births13 (Box 2). There were 126 definite cases (65%) and 69 probable cases (35%); 104 (53%) were male, 88 (45%) were female, and in three cases (2%), sex was not stated on the report. There were two neonatal deaths (at ages 1 day and 7 days) and one stillbirth. Reported gestational age ranged from 27 to 40 weeks.
The clinical and laboratory features of cases (Box 3) were similar in definite and probable cases (data not shown). No infants had cataracts or microphthalmia reported as features of symptomatic cCMV. Most features of cCMV were evident within the first 60 days of life. Sensorineural hearing loss (SNHL) was present in 51 infants (26%) and was the sole presenting feature in 34 infants, two-thirds of those with SNHL overall. In 15 cases, SNHL was diagnosed after age 60 days. Twelve of these 15 (80%) were identified retrospectively by detection of CMV PCR on their newborn screening cards,8 or from stored cord blood samples. In total, 38 infants from this group had positive CMV PCR results via testing of stored newborn screening cards or from stored blood samples.
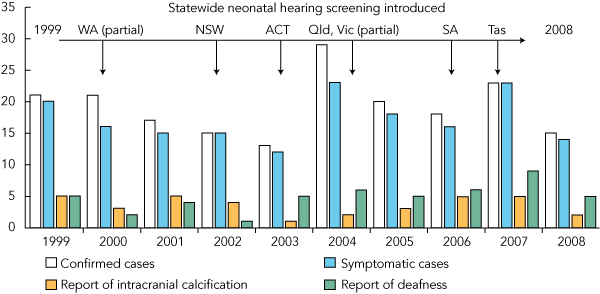
Box 1 shows cases of cCMV by year of birth, excluding four confirmed cases born before 1999, identified using CMV PCR on stored newborn screening cards.8 The overall proportion of symptomatic to total cases was not significantly different between the two study periods: 71/80 (89%) in 1999–2003 (when screening was partially introduced) and 105/115 (91%) in 2004–2009 (when screening was substantially introduced). SNHL was diagnosed in 36 of 115 cases (31%) in 2004–2009, compared with 15 of 80 cases (19%) in 1999–2003. This approached but did not reach significance (difference, 12%; 95% CI, − 0.1% to 24.1%). Analysis by state (not shown) revealed more cases identified before and after age 60 days after the introduction of neonatal hearing screening programs. There were 94 cases identified in the first half of the study (1999–2003) compared with 67 cases in the second half of the study (2004–2009) (of 161 cases where location and age of identification were available), but this also failed to attain significance. Of the 28 total cases identified after 60 days of age, 16 (57%) were identified in states after introduction of neonatal hearing screening programs, seven (25%) before introduction of such programs and five (18%) were from unknown locations. Intracranial calcification is also included in Box 1 as a recognisable radiographic predictor of poor outcome,15 which did not change significantly during the study.
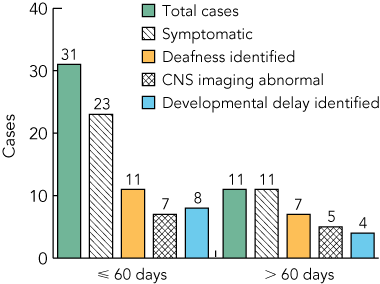
Of 31 infants identified in their first 60 days of life, nine were referred because of an antenatal diagnosis of maternal CMV and 22 infants were referred because of symptoms. Details of clinical features are shown in Box 4. All 11 infants and children identified at over 60 days of age were referred because of symptomatic CMV disease. SNHL was present in 7/11 (64%) and was the sole feature of CMV disease for three of the 42 infants (7%). SNHL was identified after the newborn period in four of 11 infants (three born before the introduction of hearing screening).
We were able to examine 23/42 infants using CMV PCR.8 Of 18 infants with a positive CMV PCR test result, 15/18 (83%) had signs of cCMV disease and 3/18 (17%) were asymptomatic. Ten of 18 (56%) had neurodevelopmental sequelae, and 7/18 (39%) had SNHL. Of those with negative PCR test results, 2/5 (40%) were symptomatic; one with isolated retinitis and one with isolated SNHL. This equated to a sensitivity of 88%, specificity of 50% and positive likelihood ratio of 1.76 for symptomatic disease with a positive CMV PCR test result. Overall, identification of cCMV was confirmed in five cases with retrospective PCR using stored newborn screening cards.
We have found that congenital cytomegalovirus infection is underreported in Australia. Based on a live birth rate of 285 000 per year13 in Australia, and a CMV birth prevalence of around 0.64%,1,10 the predicted incidence is about 1800 infected infants per year, which is substantially more than the national rates of 5–25 cases per year.16 Critically, most of these infected infants will be asymptomatic at birth and are unlikely to be identified without screening. About 13.5% (240 per year) will develop permanent sequelae.3 With an additional estimated 200–230 cases (11%–12.7%)1,3 symptomatic at birth every year, over 440 children per year may be affected by this disease in Australia, with no routine newborn screening currently in place.
Only a minority of our cases (15/195; 8%) received antiviral therapy. Although ganciclovir has been demonstrated to positively influence the clinical course of SNHL when commenced before 30 days of age in symptomatic infants,7 it is acknowledged that a safer and more practical effective treatment is needed. Valganciclovir, an oral prodrug of ganciclovir (therefore not requiring central venous access), has demonstrated pharmacokinetic equivalence with intravenous ganciclovir in neonates with symptomatic disease.17 A clinical trial is ongoing to determine whether valganciclovir improves hearing and neurological outcomes in these infants.18 When the results of this study are available, however, early identification will remain important, as cCMV cannot be reliably diagnosed after 21 days of age, except by recourse to CMV PCR testing of newborn screening cards, where available. Twenty-eight (14%) of our cases were identified after 60 days of age, which precluded consideration of evidence-based antiviral therapy to prevent hearing loss or early intervention.
In our subgroup of 42 infants for whom we undertook clinical review, one-quarter were not identified with cCMV until after 60 days of age. Neurodevelopmental problems and SNHL were common in this group, indicating the importance of considering cCMV in children with SNHL, particularly if identified late. The utility of PCR testing of newborn screening cards in diagnosing cCMV infection has been demonstrated by others.11 In our case series, positive CMV PCR test results were strongly associated with clinical disease, although negativity did not exclude disease.
During the course of this study, neonatal hearing screening was introduced in most Australian states (Ms M Wilkinson, Head of Audiology, Sydney Children’s Hospital, Randwick, personal communication, 2009) (Box 2). We found a trend of increasingly reported SNHL, which may indicate increased case ascertainment. The introduction of these screening programs was staggered across the country and, in some states, program implementation was partial, so some infants may have lived in areas without access to such screening. Children identified with SNHL are not currently routinely screened for CMV in Australia and this is arguably an important gap within the program. Neonatal hearing screening is cost-effective and allows for early intervention with hearing aids and education, which can result in functional language improvements.19 These criteria meet the World Health Organization preconditions for a screening program.20 The cost-effectiveness of incorporating cCMV screening into this algorithm urgently needs to be determined. Potential benefits include the opportunity for targeted antiviral therapy in infants with hearing loss and cCMV. This must be weighed against issues of cost and availability of timely CMV testing, as well as parental distress at identification of an infection which may or may not result in neurodevelopmental sequelae.
A routine referral program for cCMV screening in infants with hearing impairment is currently operating in Queensland, incorporated into the state’s universal neonatal hearing screening program. Internationally, there is ongoing debate about incorporation of neonatal testing for CMV, in order to allow for targeted clinical follow-up of affected infants.21-23 This view is further supported by estimates that cCMV contributes to 15%–20% of moderate-to-profound bilateral hearing loss (hearing loss of > 40 decibels) in children.24 Hearing loss in cCMV may be progressive or fluctuating, however, and more than half of infants with cCMV and SNHL may be missed by neonatal hearing screening alone.25
In centres where newborn hearing screening programs are in place, early CMV testing is important. A positive result has implications of possible hearing loss and other neurodevelopmental sequelae,3 and may allow for planned developmental follow-up as well as consideration of antiviral therapy. Furthermore, the identification of CMV is highly relevant when doctors are counselling parents about future pregnancies. Alternatively, CMV PCR testing or salivary culture could be included in routine newborn screening of all infants to allow for targeted follow-up of infants who test positive, as they are more likely to develop symptomatic disease, and SNHL in particular, beyond the neonatal age group.
2 Notifications of cCMV, January 1999 – February 2009,* and total live births in 2007, by state†
 |
|
cCMV = congenital cytomegalovirus. LB = live births. * Total cases of cCMV, 1999–2009 = 195. † Total live births, Australia, 2007 = 285 213; total fetal deaths, Australia, 2007 = 1676.14 |
Provenance: Not commissioned; externally peer reviewed.
Received 2 August 2010, accepted 14 February 2011
- Brendan J McMullan1,2
- Pamela Palasanthiran2,3
- Cheryl A Jones4,5
- Beverley M Hall6
- Peter W Robertson6
- Jonathan Howard7,2
- William D Rawlinson6,2
- 1 St Vincent’s Hospital, Sydney, NSW.
- 2 University of New South Wales, Sydney, NSW.
- 3 Sydney Children’s Hospital, Sydney, NSW.
- 4 The Children’s Hospital at Westmead, Sydney, NSW.
- 5 University of Sydney, Sydney, NSW.
- 6 South Eastern Sydney and Illawarra Area Health Service, Sydney, NSW.
- 7 Prince of Wales Hospital, Sydney, NSW.
We thank Dr Lyndall Brennan for assistance with assay development, and the APSU and reporting doctors for their contributions. APSU activities are supported by the Department of Health and Ageing; a National Health and Medical Research Council Enabling Grant (No. 402784) and Practitioner Fellowship (No. 457084, E Elliott); Discipline of Paediatrics and Child Health, Faculty of Medicine, University of Sydney; the Children’s Hospital at Westmead, and the Royal Australasian College of Physicians.
None identified.
- 1. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17: 253-276.
- 2. Zurynski Y, Cronin P, Elliott EJ. Communicable and vaccine-preventable conditions under surveillance by the APSU: 2004 update. Commun Dis Intell 2005; 29: 407-411.
- 3. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 355–363.
- 4. Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005; 5: 70.
- 5. Fowler KB, McCollister FP, Dahle AJ, et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatr 1997; 130: 624-630.
- 6. Munro SC, Trincado D, Hall B, Rawlinson WD. Symptomatic infant characteristics of congenital cytomegalovirus disease in Australia. J Paediatr Child Health 2005; 41: 449-452.
- 7. Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003; 143: 16-25.
- 8. Howard J, Hall B, Brennan L, et al. Utility of newborn screening cards for detecting CMV infection in cases of stillbirth. J Clin Virol 2009; 44: 215-218.
- 9. Barbi M, Binda S, Caroppo S, Primache V. Neonatal screening for congenital cytomegalovirus infection and hearing loss. J Clin Virol 2006; 35: 206-209.
- 10. Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol 2005; 43: 4713-4718.
- 11. Barbi M, Binda S, Primache V, et al. Cytomegalovirus DNA detection in Guthrie cards: a powerful tool for diagnosing congenital infection. J Clin Virol 2000; 17: 159-165.
- 12. McIver CJ, Jacques CF, Chow SS, et al. Development of multiplex PCRs for detection of common viral pathogens and agents of congenital infections. J Clin Microbiol 2005; 43: 5102-5110.
- 13. Australian Bureau of Statistics. Births Australia, 2007. Canberra: ABS, 2008. (ABS Cat. No. 3301.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3301.02007 (accessed May 2011).
- 14. Australian Bureau of Statistics. Perinatal deaths. Canberra: ABS, 2008. http://www.abs.gov.au/ausstats/abs@.nsf/mf/3304.0 (accessed May 2011).
- 15. Boppana SB, Fowler KB, Vaid Y, et al. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 1997; 99: 409-414.
- 16. Australian Bureau of Statistics. 2008 Year Book Australia. Canberra: ABS, 2008. (ABS Cat. No. 1301.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1301.02008?OpenDocument (accessed May 2011).
- 17. Kimberlin DW, Acosta EP, Sanchez PJ, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis 2008; 197: 836-845.
- 18. National Institute of Allergy and Infectious Diseases. Short-term vs. long-term valganciclovir therapy for symptomatic congenital CMV infections (CASG 112) [clinical trial]. http://clinicaltrials.gov/ct2/show/NCT00466817 (accessed May 2011).
- 19. Australian National Hearing Screening Committee. Australian consensus statement on universal neonatal hearing screening. Melbourne: Royal Children’s Hospital, 2002. http://www.rch.org.au/emplibrary/ccch/Con sensusFinalApr2002.pdf (accessed May 2011).
- 20. Wilson JM, Jungner YG. [Principles and practice of mass screening for disease] [Spanish]. Bol Oficina Sanit Panam 1968; 65: 281-393.
- 21. Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill 2009; 14: 26-32.
- 22. Forsgren M. Prevention of congenital and perinatal infections. Euro Surveill 2009; 14: 2-4.
- 23. Vossen A, de Vries J, van der Zeijst B. The 2008 congenital cytomegalovirus conference, 5–7 November, Centers for Disease Control and Prevention, Atlanta. Euro Surveill 2009; 14: 1-2.
- 24. Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 2008; 41: 57-62.
- 25. Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2006; 35: 226-231.





Abstract
Objectives: To report on the burden of disease in Australian infants with congenital cytomegalovirus (cCMV) infection in the era of neonatal hearing screening and improved diagnostic techniques.
Design, setting and participants: National data were collected from across Australia via the Australian Paediatric Surveillance Unit (APSU) with monthly reporting by > 1000 clinicians between January 1999 and February 2009. For each reported case, data on investigations and epidemiological and clinical features were analysed. Detailed clinical reviews were performed on 42 infants in two Sydney tertiary paediatric infectious diseases clinics.
Results: There were 195 infants with cCMV identified, including 126 definite and 69 probable cases. Of these, 175 (90%) were symptomatic and only 15 were treated with antiviral agents. Identification was delayed beyond 60 days of age in 30 cases (15%). During the period of study, neonatal hearing screening was introduced for most Australian infants. Detection of hearing loss increased from 19% of cCMV cases in 1999–2003 to 31% in 2004–2009. Of 42 infants whose cases were reviewed in detail, 33 (79%) had symptomatic disease. DNA detection of CMV, using polymerase chain reaction testing of newborn screening cards, was useful in retrospective identification, and was strongly correlated with the presence of clinical sequelae (15/18; 83%).
Conclusions: Congenital CMV is underdiagnosed, infrequently treated, and often manifests as isolated hearing loss. Delayed diagnoses both before and after the introduction of neonatal hearing screening represent missed treatment and management opportunities and are likely to lead to poorer, life-long outcomes for these children. Retrospective analysis of newborn screening cards for CMV should be undertaken for infants with sensorineural hearing loss, to identify unrecognised cCMV.