Klinefelter syndrome (KS) is a genetic condition affecting males that is most often caused by an additional X chromosome (47,XXY karyotype).1 This results in a spectrum of features ranging from azoospermia, small testes, androgen deficiency and gynaecomastia, to varying degrees of learning and behavioural difficulties2 (Box 1). The number and severity of these features are known to be highly variable between individuals.
A mean prevalence for KS of 152 per 100 000 male births was estimated from newborn screening programs in the 1960s and 1970s in several countries, including Denmark, the United States, Canada, Japan and the United Kingdom.3-10 Despite this high frequency, and features such as small testicles in adulthood, it has been estimated that less than 10% of the estimated number of affected fetuses are detected prenatally, and only 26% of live-born cases are diagnosed postnatally.11 A birth prevalence for KS of 153 per 100 000 males in Denmark has been estimated using population information and adjusting the prenatal prevalence for maternal age, as KS is an incidental finding of prenatal karyotype tests that are more commonly performed in older mothers.10 Comparison with postnatal diagnoses confirmed that only 25% of KS cases are detected.
The low diagnosis rate suggests most males with KS will not receive potentially beneficial treatments, especially androgen therapy.2 Most adult diagnoses occur during fertility assessment, beyond the ideal point for intervention.10 Detection in childhood and timely intervention may be essential for optimal medical and psychosocial outcomes in adulthood.12 Underdiagnosis may be due to men’s hesitancy about seeking medical attention, low awareness of KS among health professionals,13 and failure by health professionals to perform routine genital examinations in adult men.
A recent study suggests that the prevalence of KS may be increasing.14 The 47,XXY karyotype is almost always the result of meiotic non-disjunction during parental gamete formation, which increases with both maternal and paternal age.15,16 Given increasing parental age trends in Australia,17 and the potential clinical significance of non-diagnosis of KS, it is imperative to establish current prevalence estimates. We have used population data from Victoria to estimate local prevalence and diagnosis rates of KS.
Births in Victoria — a report on notifications of births at 20 weeks’ gestation and beyond, for which reporting is mandatory, that has been produced by the Victorian Perinatal Data Collection Unit (VPDCU) since 1983 and contains comprehensive information on mothers and babies.18
Australian Bureau of Statistics birth and population data by age, sex and state.19
Data on diagnoses of KS in Victoria were collected from all relevant sources.
The Victorian Birth Defects Register (VBDR) — a population-based surveillance system held by the VPDCU, active since 1983. Birth defects (including sex chromosome anomalies) diagnosed prenatally and postnatally up to 16 years of age are reported to the VBDR, and data were available up to and including 2005.
The Victorian Prenatal Diagnosis Database (VPDD), which contains data from cytogenetic laboratories on every chorionic villus sampling and amniocentesis procedure performed in Victoria, including indications for diagnostic testing, karyotypes and maternal variables. Data on prenatal diagnoses of KS were available for the period 1986–2006.
Two public and two private cytogenetic laboratories that undertake all karyotype testing in Victoria. These four laboratories provided data on prenatal diagnoses of KS for the period 1987–2006 and on postnatal diagnoses for 1991–2006.
The numbers of each KS karyotype identified prenatally and postnatally are shown in Box 2. The overall proportion of KS karyotypes other than 47,XXY was 14.2%. The proportion of prenatal diagnoses with non-47,XXY KS karyotypes was 20.4%, while for postnatal diagnoses it was lower, at 12.3% (P < 0.01).
A total of 152 fetuses were identified as having a KS karyotype out of 85 650 tested between 1986 and 2006. As male fetuses make up about 51% of births,17 the denominator for prevalence calculations is 43 682 (Box 3). This resulted in a crude prevalence of 348 per 100 000 males tested (95% CI, 295–408).
The mean age of Victorian women having prenatal diagnostic testing was 36.4 years in 1986 and 35.9 years in 2006 (average across the study period, 36.0 years), and the mean age of Victorian women giving birth was 27.6 years in 1986 and 30.6 years in 2006 (average across the study period, 29.6 years). Direct maternal age standardisation of the prenatal prevalence resulted in an adjusted birth prevalence of 223 per 100 000 male births (95% CI, 195–254) (Box 4).
A total of 487 cases of KS were diagnosed postnatally between 1991 and 2006 (Box 5). The cumulative postnatal diagnosis rate of KS to age 84 was estimated at 87 per 100 000 males (95% CI, 70–107). There was no significant difference between postnatal diagnosis rates for the first and second 8-year periods within the study (85 v 95 per 100 000 males; P < 0.46).
The Victorian birth prevalence rate is higher than that seen in Denmark, but falls within the range of 85 to 223 per 100 000 male births seen among the combined newborn screening surveys carried out in several countries.3-9 One factor contributing to this higher rate may be the difference in proportions of karyotypes other than 47,XXY: 14.2% in Victoria compared with 10.2% from the Danish data. However, a sensitivity analysis (data not shown) showed that, even assuming a proportion of non-47,XXY karyotypes similar to Denmark, the birth prevalence in Victoria would still be higher (194 v 153 per 100 000 males; P < 0.028). There was a significant difference (P < 0.01) between prenatal and postnatal diagnoses of these other karyotypes, possibly because live-born males with a mosaic karyotype experience fewer clinical symptoms and escape detection more often than 47,XXY males, while all karyotypes have equal likelihood of prenatal detection.
Recently, a high prevalence of KS was found in an Asian cohort (355 per 100 000 males).20 Given that more than 8% of the Australian population is of Asian descent,19 compared with 3% of the Danish population,21 this may be an additional contributing factor to the higher birth prevalence of KS seen in Victoria. Unfortunately, the ethnic backgrounds of males with KS in our dataset were not available.
Another contributing factor to the higher prevalence of KS in Victoria may be maternal age, not only at the time of prenatal diagnostic testing, but also at the time of birth. The average age of Victorian women having prenatal testing is 36.0 years, compared with 34.0 years for the Danish population,10 and these differences have been adjusted for by standardisation. However, at the population level, Victorian women giving birth are, on average, older than Danish women (29.6 years v 28.1 years for each study period). While this age difference is not large, variations between maternal age distributions may have a significant impact on the number of KS cases.
In addition, paternal age is increasing. In Australia, the average age of fathers reached an all time high of 33.1 years in 2006, with the number of men having children in their 50s increasing by around 20% over the past decade.17
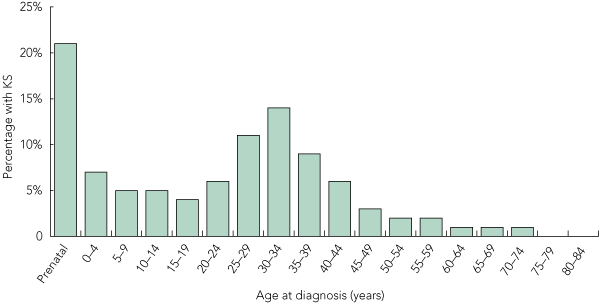
As well as a higher birth prevalence for KS, our calculations indicate a higher postnatal detection rate than that seen in Denmark10 or the UK.11 Our estimates are based on a more recent study period (1991–2006) than the Danish study (1970–2000),10 during which there has been consistent growth in access to fertility investigations in Australia.22 This may partly account for the higher detection rate and is consistent with the finding that most men are diagnosed between 25 and 39 years of age — the peak reproductive period.
The health outcomes consequent on making the diagnosis of KS are unclear, but an array of biomedical and psychosocial endpoints can be identified for which there are both empirical and evidence-based data in support of interventions. For example, up to 85% of males with KS will be testosterone-deficient after puberty,2 which may have profound medical and psychosocial impacts. Treatment may alleviate the symptoms,23 in addition to reducing condition-related morbidity and mortality.24
In an era of rapidly advancing genetic technology and greater understanding of the genetic contribution to disease, opportunities for diagnosis of KS should exist. Given the availability of treatments and interventions for KS — and the possible medical and psychosocial benefits of early diagnosis — avenues for increasing awareness, educating health professionals, and providing appropriate resources for individuals and their families must be developed further. In addition, both the risks and benefits of diagnosis through postnatal, population-based genetic screening for KS should be considered.25
1 Possible phenotypic features of Klinefelter syndrome (KS) according to life stage, and their estimated frequencies*
2 Distribution of karyotypes in prenatal (1986–2006) and postnatal (1991–2006) diagnoses of Klinefelter syndrome in Victoria, Australia
3 Annual prenatal diagnoses of Klinefelter syndrome (KS), and corresponding prevalences, 1986–2006 in Victoria, Australia
4 Maternal age standardisation of raw prenatal prevalences to estimate the overall age-adjusted prevalence of Klinefelter syndrome (KS) (per 100 000 male births) in Victoria, Australia, 1986–2006
Received 11 April 2010, accepted 19 August 2010
- Amy S Herlihy1,2,3,4
- Jane L Halliday1,5
- Megan L Cock2
- Robert I McLachlan2,3,4
- 1 Public Health Genetics, Murdoch Childrens Research Institute, Melbourne, VIC.
- 2 Andrology Australia, Melbourne, VIC.
- 3 Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC.
- 4 Clinical Andrology, Prince Henry’s Institute of Medical Research, Melbourne, VIC.
- 5 Department of Paediatrics, University of Melbourne, Melbourne, VIC.
The authors would like to thank Merilyn Riley of the VBDR, Alice Jaques and Evelyne Muggli of Public Health Genetics at the Murdoch Childrens Research Institute, and staff at the cytogenetics laboratories across Victoria for help with data collection. We are also grateful for the thoughtful suggestions of our reviewers. All authors are supported by funding from the National Health and Medical Research Council of Australia.
None identified.
- 1. Jacobs PA, Strong JA. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 1959; 183: 302-303.
- 2. Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol 2007; 4: 192-204.
- 3. Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser 1990; 26: 209-223.
- 4. Ratcliffe S. Development of children with sex chromosome abnormalities. Proc R Soc Med 1976; 69: 3.
- 5. Hamerton JL, Canning N, Ray M, Smith S. A cytogenetic survey of 14,069 newborn infants. I. Incidence of chromosome abnormalities. Clin Genet 1975; 8: 223-243.
- 6. Bochkov NP, Kuleshov NP, Chebotarev AN, et al. Population cytogenetic investigation of newborns in Moscow. Humangenetik 1974; 22: 139-152.
- 7. Higurashi M, Iijima K, Ishikawa N, et al. Incidence of major chromosome aberrations in 12,319 newborn infants in Tokyo. Hum Genet 1979; 46: 163-172.
- 8. Leonard MF, Schowalter JE, Landy G, et al. Chromosomal abnormalities in the New Haven newborn study: a prospective study of development of children with sex chromosome anomalies. Birth Defects Orig Artic Ser 1979; 15: 115-159.
- 9. Maclean N, Harnden DG, Brown WM, et al. Sex-chromosome abnormalities in newborn babies. Lancet 1964; 1: 286-290.
- 10. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003; 88: 622-626.
- 11. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn 1997; 17: 363-368.
- 12. Simpson JL, De La Cruz F, Swerdloff RS, et al. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med 2003; 5: 460-468.
- 13. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet 2004; 364: 273-283.
- 14. Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet 2008; 16: 163-170.
- 15. Ferguson-Smith MA, Yates JR. Maternal age specific rates for chromosome aberrations and factors influencing them. Report of a collaborative European study on 52,965 amniocenteses. Prenat Diagn 1984; 4: 5-44.
- 16. De Souza E, Morris JK; EUROCAT working group. Case-control analysis of paternal age and trisomic anomalies. Arch Dis Child 2010; Jun 28.
- 17. Australian Bureau of Statistics. Births Australia 2006. Canberra: ABS, 2006: (ABS Cat. No. 3301.0.)
- 18. Davey M-A, Taylor O, Oats JJN, et al. Births in Victoria 2005 and 2006. Melbourne: Victorian Perinatal Data Collection Unit, Department of Human Services, 2008.
- 19. Australian Bureau of Statistics. Year book Australia, 2009–10. (ABS Cat. No. 1301.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/796378C9B98D7F1CCA 25773700177E5E?opendocument (accessed Nov 2009).
- 20. Coffee B, Keith K, Albizua I, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet 2009; 85: 503-514.
- 21. Denmark S. [Population statistics, 2009] [Danish]. http://www.statistikbanken.dk (accessed Nov 2009).
- 22. Wang YA, Dean JH, Badgery-Parker T, Sullivan EA. Assisted reproduction technology in Australia and New Zealand 2006. Sydney: Australian Institute of Health and Welfare, National Perinatal Statistics Unit; 2006. (AIHW Cat. No. PER 43.)
- 23. Nielsen J, Pelsen B, Sorensen K. Follow-up of 30 Klinefelter males treated with testosterone. Clin Genet 1988; 33: 262-269.
- 24. Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocr Metab 2006; 91: 1254-1260.
- 25. Herlihy AS, Halliday J, McLachlan RI, et al. Assessing the risks and benefits of diagnosing genetic conditions with variable phenotypes through population screening: Klinefelter syndrome as an example. J Community Genet 2010; 1: 41-46.






Abstract
Objective: To determine the prevalence and diagnosis rates of Klinefelter syndrome (KS) in Victoria, Australia, and compare these to previous international findings.
Design, setting and participants: A Victorian population-based descriptive study of all cytogenetic examinations resulting in a diagnosis of KS, including prenatal diagnoses from 1986 to 2006 and postnatal diagnoses from 1991 to 2006.
Main outcome measures: Birth prevalence and diagnosis rates of KS.
Results: The birth prevalence of KS in Victoria is estimated to be 223 per 100 000 males (95% CI, 195–254), with about 50% of cases remaining undiagnosed.
Conclusions: KS may be occurring more frequently than has been reported previously, yet many cases remain undiagnosed. Our results highlight the need for increased awareness leading to timely detection.