Antimicrobial resistance is a major threat to the great advances in treatment of infectious diseases over the past 40 years.1 The relationship between antimicrobials and resistant organisms is complex, encompassing selection and dissemination of resistance determinants between humans and bacterial hosts. Despite difficulties in proving a cause–effect relationship, there is good evidence that overuse and inappropriate use of antimicrobials lead to emergence and dissemination of resistant organisms, with studies showing that resistance rises with increased antimicrobial use and falls after reduced use.1-3 Patients with infections due to resistant bacteria have poorer outcomes, experiencing delayed recovery, treatment failure and even death.4 Inappropriate use of antimicrobials also increases the risk of patient harm from adverse effects such as Clostridium difficile infection, and increases costs to health care and society. Prudent use of antimicrobials is considered central to the control of resistance, and active surveillance of antimicrobial usage is paramount.
Changing antimicrobial use in hospitals is complex and challenging and requires an organised approach, such as an antimicrobial management program, also termed antimicrobial stewardship (AMS). AMS involves a systematic approach to optimising antimicrobial use. Successful hospital AMS programs have been shown to improve the appropriateness of antimicrobial use, and to reduce institutional resistance rates and, in turn, morbidity and mortality.5-10 Together with infection control, hand hygiene and surveillance, AMS is considered a key strategy in local and national programs to decrease preventable health care-associated infections. When supported by hospital management, a decrease in inappropriate use, improved patient outcomes and savings in health care costs can be achieved.5,6,8,9
Hospital AMS programs include a range of different interventions aimed at improving antimicrobial prescribing. One of the essential components of an AMS program is monitoring antimicrobial usage11 to:
identify trends in prescribing that require further investigation through targeted audits;
measure the effect of stewardship activities, including cost savings; and
provide feedback to prescribers (one of the most effective interventions to influence prescribing behaviour).
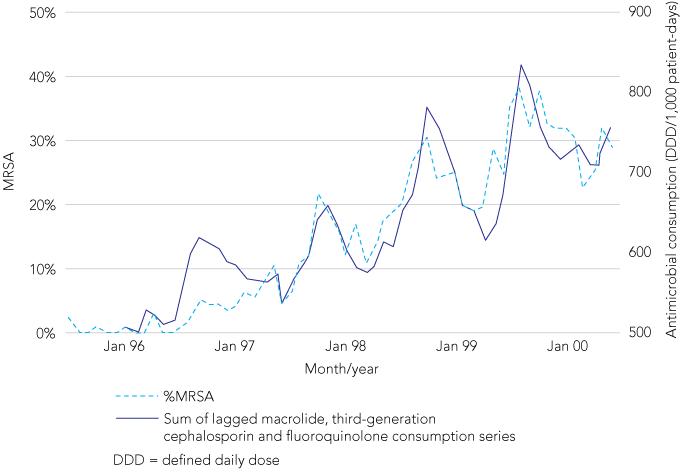
The monitoring of antimicrobial usage is also critical to understanding antimicrobial resistance by linking patterns of usage with the emergence of resistant organisms. Box 1 provides an example of the temporal relationship between the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and the use of antimicrobial agents known to induce methicillin resistance.
There are two main methods of antimicrobial data collection: patient-level surveillance and population surveillance.13
Comprehensive data at individual patient level are not available from most hospitals in Australia, and aggregate data from issues to wards combined with individual patient dispensing records are most commonly used.1 Here, we discuss how population surveillance data can be used to drive safety and quality improvement in hospital practice.
Local-level data can be used to:
provide regular feedback enabling institutions to examine their antimicrobial usage rates over time and to target areas of high antimicrobial usage for local intervention programs (see Case study 1 and Case study 2); and
initiate and evaluate the effect of programs in addressing the incidence of resistant organisms and associated patient morbidity, mortality and health care costs (see Case study 3 and Case study 4).
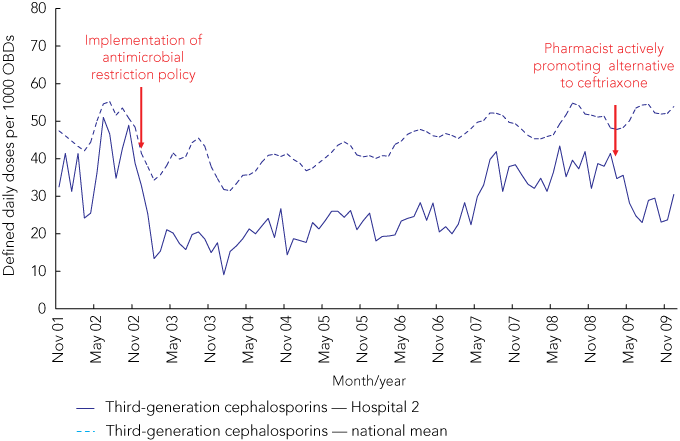
High usage of third-generation cephalosporins in a major South Australian metropolitan hospital was noted in 2002 through data collection and analysis by the South Australian Antimicrobial Utilisation Surveillance Program. The hospital implemented an antimicrobial restriction policy in January 2003. The intervention focused on community-acquired pneumonia treatment protocols, which had been identified through pharmacy audit as an area of inappropriate use of ceftriaxone. The usage of ceftriaxone decreased significantly following the implementation of the new policy, and this level of use was sustained for about 4 years. However, ceftriaxone use then rose again. A second intervention commenced in 2009, in which an AMS pharmacist actively promoted the use of alternative agents and instituted a program of switching to oral therapy within agreed periods according to the patient’s condition. These strategies resulted in a decline in ceftriaxone usage (Box 3).
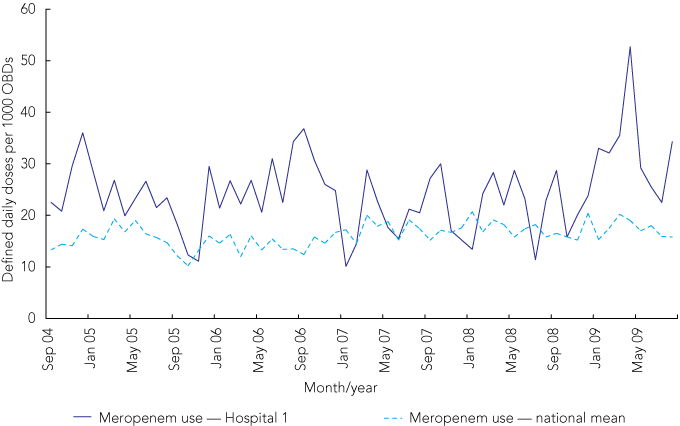
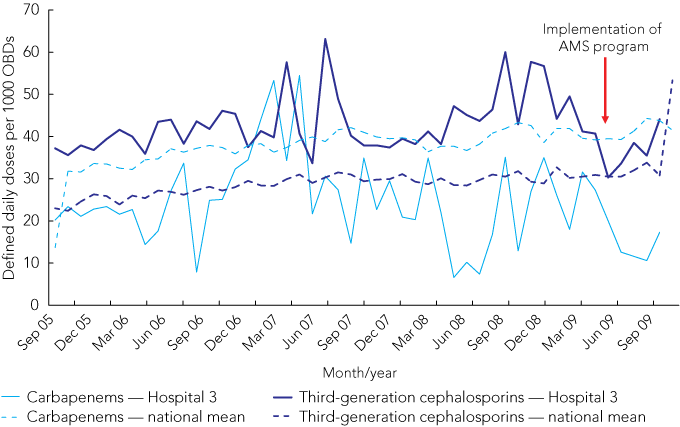
Since the electronic antimicrobial approval system commenced in May 2009, there has been a decline in the usage of carbapenems and third-generation cephalosporins (Box 4). The use of these broad-spectrum antimicrobials is known to be linked with development of multidrug-resistant organisms and an increase in incidence of C. difficile infection.14
Several published studies indicate that AMS programs cover at least their costs and can be financially self-supporting.5,6 Case study 4 demonstrates the use of comparative antimicrobial usage data to determine the savings in drug costs attributable to the hospital’s AMS program.
examine trends in hospital antimicrobial use at state and national levels as the basis for larger-scale interventions to rationalise hospital antimicrobial prescribing;
provide an Australian peer-group benchmark, and to enable comparison with international data (it is known that aggregate use of antimicrobials is higher in Australia than that reported by several European surveillance programs15); and
provide longitudinal antimicrobial usage data which may be used to demonstrate links between antimicrobial use and resistance.
- Vicki McNeil1
- Marilyn Cruickshank2
- Margaret Duguid2
- 1 Antimicrobial Utilisation Surveillance Programs, Infection Control Service, Communicable Disease Control Branch, SA Health, Adelaide, SA.
- 2 Australian Commission on Safety and Quality in Health Care, Sydney, NSW.
We acknowledge the assistance of contributors to the NAUSP, in particular the case study contributors. The NAUSP is funded by the Australian Government Department of Health and Ageing and conducted by the Infection Control Service, Communicable Diseases Control Branch, SA Health.
None identified.
- 1. Cruickshank M, Ferguson J, editors. Reducing harm to patients from health care associated infection: the role of surveillance. Sydney: Australian Commission on Safety and Quality in Health Care, 2008.
- 2. Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998; 279: 365-370.
- 3. Richard P, Delangle M, Merrien D, et al. Fluoroquinolone use and fluoroquinolone resistance: is there an association? Clin Infect Dis 1994; 19: 54-59.
- 4. Roberts R, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antimicrobial stewardship. Clin Infect Dis 2009; 49: 1175-1184.
- 5. Dellit H, Owens R, McGowan J, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159-177.
- 6. MacDougall C, Polk R. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 2005; 18: 638-656.
- 7. Drew R, White R, MacDougall C, et al. Insights from the Society of Infectious Diseases Pharmacists on Antimicrobial Stewardship Guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Pharmacotherapy 2009; 29: 593-607.
- 8. Fishman N. Antimicrobial stewardship. Am J Infect Control 2006; 34: S55-S63.
- 9. Carling P, Fung T, Killion A, et al. Favourable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol 2003; 24: 699-706.
- 10. Davey P, Brown E, Fenelon L, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005; (4): CD003543.
- 11. Australian Commission on Safety and Quality in Health Care. Windows into safety and quality in health care 2009. Sydney: ACSQHC, 2009.
- 12. Monnet D, MacKenzie F, López-Lozano J, et al. Antimicrobial drug use and methicillin-resistant Staphylococcus aureus, Aberdeen, 1996–2000. Emerg Infect Dis 2004; 10: 1432-1441.
- 13. Kritsotakis E, Gikas A. Surveillance of antibiotic use in hospitals: methods, trends and targets. Clin Microbiol Infect 2006; 12: 701-704.
- 14. Gerding D. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin Infect Dis 2004; 38: 646-648.
- 15. National Antimicrobial Utilisation Surveillance Program. Annual report 2008–2009. Adelaide: NAUSP, 2009. http://www.health.sa.gov.au/INFECTIONCONTROL/Default.aspx?PageContentID=65&tabid=199 (accessed Mar 2010).





Abstract
The National Antimicrobial Utilisation Surveillance Program (NAUSP) collects aggregate data from hospitals in all Australian states and provides reports of monthly hospital inpatient antimicrobial usage to contributing hospitals.
These data provide an Australian peer-group benchmark; hospitals can compare their usage with similar hospitals and identify areas of antimicrobial use that require more indepth analysis.
Overall high usage has been used by hospitals and area health services as a stimulus for initiation or expansion of antimicrobial stewardship programs.
High use of particular classes of antimicrobials has triggered individual drug audits and been used to tailor interventions.
Longitudinal antimicrobial usage data have been used by hospitals to measure the effects of antimicrobial stewardship strategies and provide feedback to prescribers.