Pancreatic exocrine insufficiency (PEI) can occur as a consequence of numerous diseases, including pancreatic cancer, chronic pancreatitis, cystic fibrosis and/or gastrointestinal surgery (particularly pancreatectomy). Although local and international guidelines have been developed for diagnosis and management of these individual disease states,1-4 none have focused specifically on diagnosis and management of PEI across all disease states.
The recommendations presented here were developed by the Australasian Pancreatic Club (Box 1) from evidence sourced from Medline, EMBASE and the Cochrane library. Each of the findings and recommendations were categorised according to the available level of evidence (Box 2).5 The level of evidence is indicated in brackets after each recommendation. Where evidence could not be found, the opinion of the group (denoted as Level 5 evidence) was used to provide guidance. We hope that, where evidence is lacking, these recommendations will serve as an impetus for future research.
Pancreatic enzymes play a critical role in macronutrient digestion. Their secretion is predominantly stimulated by exposure of the duodenal mucosa to nutrients. PEI occurs when amounts of enzymes secreted into the duodenum in response to a meal are insufficient to maintain normal digestive processes. There are three main reasons for insufficiency of pancreatic enzymes:6
Reduced capacity of the pancreas to synthesise enzymes due to loss of or injury to the pancreatic parenchyma;
Reduced stimulation of enzyme production due to postprandial asynchrony; and
Impaired delivery of enzymes to the duodenum due to obstruction of the pancreatic duct.
Because of the high reserve capacity of the pancreas and compensatory mechanisms that partly substitute for the loss of pancreatic enzymes, clinical symptoms of PEI do not usually manifest until duodenal lipase levels fall below 5%–10% of normal postprandial levels.6,7
The main clinical consequence of PEI is fat maldigestion and malabsorption, resulting in steatorrhoea. Steatorrhoea is characterised by frothy, foul-smelling and buoyant stools, due to their high fat content. Other symptoms may also include abdominal pain, flatulence and weight loss in adults, or lack of weight gain in children. If left untreated, fat maldigestion may lead to low circulating levels of micronutrients, fat-soluble vitamins and lipoproteins, which have been related to high morbidity because of increased risk of malnutrition-related complications and cardiovascular events.8,9
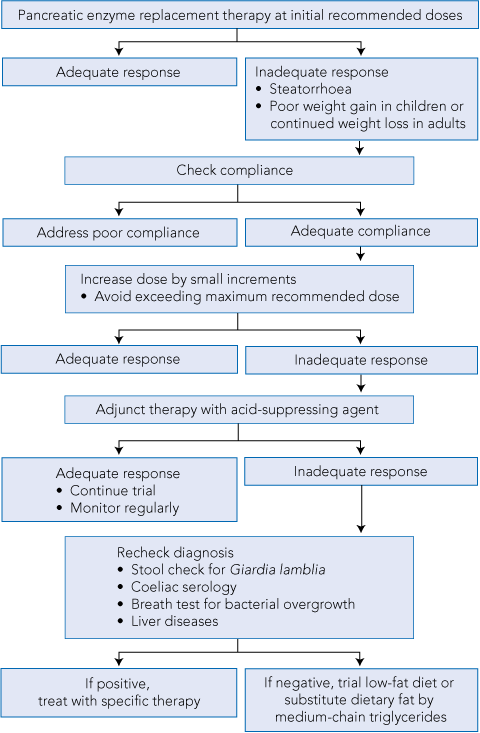
The primary treatment goal for PEI is to eliminate maldigestion and malabsorption and maintain adequate nutrition. A treatment algorithm is proposed in Box 3.
The relationship between dose of pancreatic enzymes required and the presence of malabsorption and maldigestion is not linear. Therefore, patients should start on the lowest recommended dose of pancreatic enzymes, which should then be increased and titrated to the lowest effective dose (Level 5; Box 4). Initially, PERT was thought to be free of major side effects, but there is an increasing recognition of fibrosing colonopathy and its association with very high doses of oral pancreatic enzymes in patients with cystic fibrosis.13-15 For this reason, maximum dose recommendations have been made (Box 4).
Timing related to meals can influence the effectiveness of pancreatic enzymes. If taken before the meal, enzymes may be emptied from the stomach before the meal is emptied. If enzymes are taken after the meal, some of the meal may be emptied before the enzymes. In both cases, digestion may be incomplete. Enzymes should be taken with the meal to ensure adequate mixing with the chyme (Level 2b).16
PERT reduces maldigestion due to PEI and improves the nutritional status of patients. But, in spite of adequate enzyme doses, patients may continue to experience symptoms (in particular, steatorrhoea). This may contribute to malnutrition and weight loss.17
Orally administered pancreatic enzymes can be inactivated by gastric acid.18 In theory, drug therapy that reduces gastric acid may improve the effectiveness of PERT. Acid-suppressing agents may be useful for patients who continue to experience symptoms of PEI despite high-dose enzyme therapy (Level 1b).19-21
Weight loss in adults or lack of weight gain in children is common in PEI because of fat malabsorption and the patient’s fear of eating (due to exacerbation of symptoms such as abdominal pain and flatulence). Ensuring adequate growth in children and preventing weight loss in adults is paramount. Historically, dietary fat intake has been restricted in patients with PEI to minimise fat malabsorption and reduce steatorrhoea. However, low-fat diets are lower in total energy content, and restricting fat intake also reduces intake of fat-soluble vitamins, which are already malabsorbed in people with PEI. Today, fat restriction is no longer recommended (Level 5). Normal and high-fat diets have been successfully used in combination with adequate PERT.22,23 In children with cystic fibrosis, titration of meal fat content with PERT has evolved (Box 4). As no similar recommendations have been formulated for adults, adult patients need to be educated about how to estimate fat content in meals.
The use of medium-chain triglycerides in the dietary management of PEI is controversial. The potential benefit of medium-chain over long-chain triglycerides is their higher energy value. Few human studies have evaluated the therapeutic efficacy of medium-chain trigylcerides in clinical practice, and the results do not suggest any clear nutritional advantage over the usual long-chain triglycerides when pancreatic enzymes are used.24-26 Medium-chain triglycerides are poorly tolerated in many patients and can induce side effects such as abdominal pain, nausea and diarrhoea. They may be trialled in patients whose symptoms persist despite enzyme therapy, or when weight gain is very difficult (Level 5).
With any PEI, alcohol abstinence is crucial (Level 3a), as alcohol inhibits gastric lipase secretion, and therefore contributes to fat malabsorption. With time, alcohol consumption can cause more severe and rapid deterioration of pancreatic function.27
Acute pancreatitis is an inflammatory disease most commonly caused by gallstones or alcohol abuse28 and is associated with significant morbidity and mortality.3 While there is no evidence to support the use of PERT during the initial stages of acute pancreatitis (Level 1b),29 the data do support the fact that some patients have pancreatic exocrine dysfunction for a period of time after acute pancreatitis. Therefore, patients should be monitored for PEI for at least 6–18 months and treated with oral pancreatic enzymes as indicated (Level 2b).30-49 As the length of time for recovery of exocrine function appears to depend on the severity of the episode, it may be prudent to supplement patients recovering from an acute necrotising attack with oral pancreatic enzymes and then evaluate exocrine function later in the recovery period (Level 5).
Chronic pancreatitis is characterised by progressive and irreversible damage to both the exocrine and endocrine components of the pancreas.50 Alcohol is considered the primary cause, accounting for 60%–70% of all cases.51,52 People with alcoholic pancreatitis generally develop PEI within 5–6 years of disease onset.53 Dietary counselling, coupled with PERT, in patients with chronic pancreatitis not only improves the symptoms of PEI (Level 3a),22,54,55 but can also significantly improve patients’ quality of life (Level 4).56
The role of PERT in reducing pain in patients with chronic pancreatitis remains unclear.56-58 The American Gastroenterological Association recommends a trial of high-dose pancreatic enzymes coupled with acid suppression therapy before proceeding with continuous use of narcotics or invasive treatment.59
Pancreatitis in children often has a different aetiology and natural history than in adults.60 There is no evidence for the use of PERT for treatment of acute pancreatitis in children (Level 5). Supplemental enzymes should be used in patients with chronic pancreatitis and documented PEI (Level 5). In patients with painful chronic pancreatitis, PERT may be trialled for pain relief even in the absence of documented PEI (Level 1b).56,61-64
Cystic fibrosis is a common lethal genetic disorder that is primarily diagnosed at birth through newborn screening.2,65 About 85% of cystic fibrosis patients have pancreatic insufficiency by early childhood.12 Prolonged, untreated PEI is associated with a poorer prognosis in the long term.66 Good nutritional management of patients with cystic fibrosis can prevent growth failure and chronic malnutrition (Level 1b).67,68 PERT is indicated for those with documented fat malabsorption or PEI. Branded enzyme preparations should be used to ensure high quality and efficacy (Level 2a).69
Bowel surgery involves procedures to remove a diseased part of the large or small intestine. Patients can develop PEI after bowel resection, particularly those who have undergone extensive small bowel surgeries (Level 2a).70,71 Theoretically, PERT could be prescribed with gastric acid suppression therapy to enable gastric digestion of nutrients and increase the delivery of digestive products to the small bowel (Level 5).
Gastrectomy is performed most commonly to treat cancer, bleeding gastric ulcers, polyps and perforations of the stomach wall. Most patients who have undergone partial or total gastrectomy develop PEI, although the causes and underlying mechanisms remain unclear.72-74 These patients can benefit from PERT after surgery (Level 2a).75,76 Adequate and appropriate enzyme substitution may reduce maldigestion and contribute to improvement in postgastrectomy nutritional status.10,73,77,78
Pancreatectomy is a treatment option for both benign and malignant diseases of the pancreas. Different pancreatic resections are associated with varying risk of developing PEI. All patients who have undergone pancreatic surgery should be screened for PEI (Level 3a).79-82 PEI should be suspected in all patients who have had major pancreatic resection. Although difficult to diagnose in these patients, it is important to try to establish the presence of PEI, as long-term oral PERT can significantly improve the quality of life of these patients (Level 4).56,83,84
About 90% of patients with pancreatic cancer have weight loss at the time of diagnosis.85 Weight loss may be exacerbated by maldigestion and malabsorption, as a result of destroyed pancreatic tissue reducing the availability of pancreatic enzymes.86,87 This results in PEI with associated steatorrhoea.23 PERT can be used to treat PEI in patients with unresectable pancreatic cancer to help maintain weight and improve overall quality of life (Level 2a).22,23,87
PEI is common in patients with untreated coeliac disease, but is reversible in those who have a good clinical response to gluten withdrawal.88,89 Patients with persisting symptoms after gluten withdrawal should have their pancreatic exocrine function assessed (Level 2b).90-95 Those found to have PEI should be treated with PERT (Level 2b).92,93,95
Supplementation with pancreatic enzymes may also benefit infants with coeliac disease in the period immediately after diagnosis, irrespective of pancreatic function (Level 1b).96
As the exocrine and endocrine portions of the pancreas are linked anatomically and physiologically, any disease affecting one part has the potential to affect the other part.97 PEI is frequently associated with both type 1 and type 2 diabetes mellitus (Level 2b).98-102 In about 60% of patients,103 the PEI can cause characteristic steatorrhoea, and treatment with PERT may be indicated (Level 4).54,102,104,105
Fat malabsorption is a frequent problem in patients with HIV infection. Steatorrhoea may be due to PEI in about 30% of cases (Level 3b).106-110 Treatment with PERT can reduce faecal fat loss and relieve the symptoms of steatorrhoea in HIV-infected patients with fat malabsorption (Level 4).109,110
Irritable bowel syndrome is a common condition, characterised by abdominal pain, bloating and abnormal bowel habit. PEI may occur in patients with diarrhoea-predominant irritable bowel syndrome (Level 2b).111,112 Treatment with PERT may reduce diarrhoea and abdominal pain (Level 3b).111,113
Through the development of these recommendations it has become apparent that there is a lack of good quality clinical evidence for many areas in the management of PEI. In such instances, recommendations are based primarily on clinical experience rather than clinical data. PERT remains the mainstay treatment for PEI. However, it is difficult to draw conclusions from a number of clinical trials because of significant differences in study design. There is no standardised method for assessing pancreatic exocrine function, and thus no defined criteria for diagnosing PEI. Furthermore, over the past 50 years, PERT has evolved, and numerous different formulations have been developed, evaluated and marketed during that time. In addition to these methodological issues, PEI has diverse aetiologies and patients with PEI are a heterogeneous population. Clearly, further research is needed to optimise patient management. A copy of the full recommendations is available at the Australasian Pancreatic Club website (http://www.pancreas.org.au).
1 Development of the recommendations
These recommendations were developed on the initiative of a group of senior members of the Australasian Pancreatic Club. It was done on the realisation that emerging data supported the use of pancreatic enzyme replacement therapy in a manner that had previously not been used, and that patients eligible for this therapy may not have been receiving it in an effective manner. The clinicians met on two occasions, firstly to define the outline of the document, set tasks and develop a mechanism for agreeing on a consensus statement that would make up the recommendations. A decision was made to use the Sackett system5 for classifying the quality of evidence derived from a wide search of the literature. As the project evolved, communication between members of the group occurred electronically so that consensus was reached. At the second meeting, the recommendations were discussed, and unanimous agreement was reached on each of them. Sheryl Perkin (a medical writer for Grey Healthcare) was engaged to act as an assistant to source relevant publications. These were reviewed by the panel members and summarised in an all-inclusive document. Sheryl also acted as a ghost writer for the original document. The document was then summarised into a manuscript submitted to the Journal.
- James Toouli1
- Andrew V Biankin2
- Mark R Oliver3
- Callum B Pearce4
- Jeremy S Wilson5
- Nicholas H Wray6
- 1 Department of Surgery, Flinders Medical Centre, Adelaide, SA.
- 2 Garvan Institute of Medical Research, Sydney, NSW.
- 3 Department of Gastroenterology and Clinical Nutrition, Royal Children’s Hospital, Melbourne, VIC.
- 4 School of Medicine and Pharmacology, Fremantle Hospital Unit, University of Western Australia, Perth, WA.
- 5 South Western Sydney Clinical School, University of New South Wales, Liverpool Hospital, Sydney, NSW.
- 6 Flinders Private Hospital, Adelaide, SA.
We gratefully acknowledge the assistance of Sheryl Perkin in researching the literature and in medical writing of the recommendations and manuscript. The activities of the Australasian Pancreatic Club’s committee were supported by an unrestricted educational grant from Solvay Pharmaceuticals.
The Australasian Pancreatic Club has received an unrestricted educational grant from Solvay Pharmaceuticals to support the formation of a committee to develop evidence-based recommendations. The South Western Sydney Clinical School, University of New South Wales, has received an unrestricted educational grant from Solvay Pharmaceuticals to support time spent in preparing the manuscript. James Toouli, Andrew Biankin, Mark Oliver, Callum Pearce and Nicholas Wray have received payment from Solvay Pharmaceuticals to attend meetings relating to preparation of the recommendations.
- 1. Pancreas Study Group, Chinese Society of Gastroenterology. Guidelines for the diagnosis and treatment of chronic pancreatitis (Nanjing, 2005). Chin J Dig Dis 2005; 6: 198-201.
- 2. Stapleton D, Ash C, King S, et al. Australasian clinical practice guidelines for nutrition in cystic fibrosis. 2006. http://www.cysticfibrosis.org.au/pdf/CF_Nutrition_Guidelines.pdf (accessed Feb 2010).
- 3. Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101: 2379-2400.
- 4. Pancreatic Section, British Society of Gastroenterology; Pancreatic Society of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; Royal College of Pathologists; Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005; 54 Suppl 5: v1-v16.
- 5. Sackett DL, Straus SE, Richardson WS, et al. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Philadelphia: Churchill Livingstone, 2000.
- 6. Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005; 54 Suppl 6: vi1-vi28.
- 7. DiMagno EO, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med 1973; 288: 813-815.
- 8. Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep 2007; 9: 116-122.
- 9. Montalto G, Soresi M, Carroccio A, et al. Lipoproteins and chronic pancreatitis. Pancreas 1994; 9: 137-138.
- 10. Layer P, Keller J, Lankisch PG. Pancreatic enzyme replacement therapy. Curr Gastroenterol Rep 2001; 3: 101-108.
- 11. Anthony H, Collins CE, Davidson G, et al. Pancreatic enzyme replacement therapy in cystic fibrosis: Australian guidelines. Pediatric Gastroenterological Society and the Dietitians Association of Australia. J Paediatr Child Health 1999; 35: 125-129.
- 12. Baker SS. Delayed release pancrelipase for the treatment of pancreatic exocrine insufficiency associated with cystic fibrosis. Ther Clin Risk Manag 2008; 4: 1079-1084.
- 13. Smyth RL, van Velzen D, Smyth AR, et al. Strictures of ascending colon in cystic fibrosis and high-strength pancreatic enzymes. Lancet 1994; 343: 85-86.
- 14. Pawel BR, de Chadarévian JP, Franco ME. The pathology of fibrosing colonopathy of cystic fibrosis: a study of 12 cases and review of the literature. Hum Pathol 1997; 28: 395-399.
- 15. Stevens JC, Maguiness KM, Hollingsworth J, et al. Pancreatic enzyme supplementation in cystic fibrosis patients before and after fibrosing colonopathy. J Pediatr Gastroenterol Nutr 1998; 26: 80-84.
- 16. Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, et al. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther 2005; 21: 993-1000.
- 17. Ng SM, Jones AP. Drug therapies for reducing gastric acidity in people with cystic fibrosis. Cochrane Database Syst Rev 2003; (2): CD003424.
- 18. Zentler-Munro PL, Fine DR, Batten JC, Northfield TC. Effect of cimetidine on enzyme inactivation, bile acid precipitation, and lipid solubilisation in pancreatic steatorrhoea due to cystic fibrosis. Gut 1985; 26: 892-901.
- 19. Robinson P, Sly PD. Placebo-controlled trial of misoprostol in cystic fibrosis. J Pediatr Gastroenterol Nutr 1990; 11: 37-40.
- 20. Heijerman HG, Lamers CB, Bakker W, Dijkman JH. Improvement of fecal fat excretion after addition of omeprazole in pancrease in cystic fibrosis is related to residual exocrine function of the pancreas. Dig Dis Sci 1993; 38: 1-6.
- 21. Proesmans M, De Boeck K. Omeprazole, a proton pump inhibitor, improves residual steatorrhoea in cystic fibrosis patients treated with high dose pancreatic enzymes. Eur J Pediatr 2003; 162: 760-763.
- 22. Safdi M, Bekal PK, Martin S, et al. The effects of oral pancreatic enzymes (Creon 10 capsule) on steatorrhoea: a multicenter, placebo-controlled, parallel group trial in subjects with chronic pancreatitis. Pancreas 2006; 33: 156-162.
- 23. Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998; 42: 92-96.
- 24. Caliari S, Benini L, Sembenini C, et al. Medium-chain triglyceride absorption in patients with pancreatic insufficiency. Scand J Gastroenterol 1996; 31: 90-94.
- 25. Durie PR, Newth CJ, Forstner GG, Gall DG. Malabsorption of medium-chain triglycerides in infants with cystic fibrosis. Correction with pancreatic enzyme supplement. J Pediatr 1980; 96: 862-864.
- 26. Caliari S, Benini L, Bonfante F, et al. Pancreatic extracts are necessary for the absorption of elemental and polymeric enteral diets in severe pancreatic insufficiency. Scand J Gastroenterol 1993; 28: 749-752.
- 27. Gullo L, Barbara L, Labò G. Effect of cessation of alcohol use on the course of pancreatic dysfunction in alcoholic pancreatitis. Gastroenterology 1988; 95: 1063-1068.
- 28. Lankisch PG. Epidemiology of acute pancreatitis. In: Buechler MW, Friess H, Malfertheiner P, Uhl W, editors. Acute pancreatitis: novel concepts in biology and therapy. London: Blackwell Science, 1999: 145-153.
- 29. Patankar RV, Chand R, Johnson CD. Pancreatic enzyme supplementation in acute pancreatitis. HPB Surg 1995; 8: 159-162.
- 30. Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology 2003; 3: 303-308.
- 31. Migliori M, Pezzilli R, Tomassetti P, Gullo L. Exocrine pancreatic function after alcoholic or biliary acute pancreatitis. Pancreas 2004; 28: 359-363.
- 32. Domínguez-Muñoz JE, Pieramico O, Büchler M, Malfertheiner P. Exocrine pancreatic function in the early phase of human acute pancreatitis. Scand J Gastroenterol 1995; 30: 186-191.
- 33. Pezzilli R, Simoni P, Casadei R, Morselli-Labate AM. Exocrine pancreatic function during the early recovery phase of acute pancreatitis. Hepatobiliary Pancreat Dis Int 2009; 8: 316-319.
- 34. Bozkurt T, Maroske D, Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepatogastroenterology 1995; 42: 55-58.
- 35. Mitchell CJ, Playforth MJ, Kelleher J, McMahon MJ. Functional recovery of the exocrine pancreas after acute pancreatitis. Scand J Gastroenterol 1983; 18: 5-8.
- 36. Pelli H, Lappalainen-Lehto R, Piironen A, et al. Pancreatic damage after the first episode of acute alcoholic pancreatitis and its association with the later recurrence rate. Pancreatology 2009; 9: 245-251.
- 37. Bavare C, Prabhu R, Supe A. Early morphological and functional changes in pancreas following necrosectomy for acute severe necrotizing pancreatitis. Indian J Gastroenterol 2004; 23: 203-205.
- 38. Sabater L, Pareja E, Aparisi L, et al. Pancreatic function after severe acute biliary pancreatitis: the role of necrosectomy. Pancreas 2004; 28: 65-68.
- 39. Pareja E, Artigues E, Aparisi L, et al. Exocrine pancreatic changes following acute attack of biliary pancreatitis. Pancreatology 2002; 2: 478-483.
- 40. Malecka-Panas E, Juszynski A, Wilamski E. Acute alcoholic pancreatitis does not lead to complete recovery. Mater Med Pol 1996; 28: 64-68.
- 41. Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP 2006; 7: 447-453.
- 42. Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg 2001; 167: 281-286.
- 43. Gupta R, Wig JD, Bhasin DK, et al. Severe acute pancreatitis: the life after. J Gastrointest Surg 2009; 13: 1328-1336.
- 44. Angelini G, Pederzoli P, Caliari S, et al. Long-term outcome of acute necrohemorrhagic pancreatitis. A 4-year follow-up. Digestion 1984; 30: 131-137.
- 45. Reszetow J, Hac S, Dobrowolski S, et al. Biliary versus alcohol related infected pancreatic necrosis: similarities and differences in the follow-up. Pancreas 2007; 35: 267-272.
- 46. Connor S, Alexakis N, Raraty MG, et al. Early and late complications after pancreatic necrosectomy. Surgery 2005; 137: 499-505.
- 47. Tsiotos GG, Luque-de León E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg 1998; 85: 1650-1653.
- 48. Tzovaras G, Parks RW, Diamond T, Rowlands BJ. Early and long-term results of surgery for severe necrotising pancreatitis. Dig Surg 2004; 21: 41-46.
- 49. Endlicher E, Völk M, Feuerbach S, et al. Long-term follow-up of patients with necrotizing pancreatitis treated by percutaneous necrosectomy. Hepatogastroenterology 2003; 50: 2225-2228.
- 50. DiMagno EP, Layer P, Clain JE. Chronic pancreatitis. In: Go VL, DiMagno EP, Gardner JD, et al, editors. The pancreas: biology, pathobiology and disease. 2nd ed. New York: Plenum Press, 1993: 665–706.
- 51. Tandon RK, Sato N, Garg PK; Consensus Study Group. Chronic pancreatitis: Asia–Pacific consensus report. J Gastroenterol Hepatol 2002; 17: 508-518.
- 52. Dumasy V, Delhaye M, Cotton F, Deviere J. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol 2004; 99: 1350-1354.
- 53. Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 2007; 132: 1557-1573.
- 54. O’Keefe SJ, Cariem AK, Levy M. The exacerbation of pancreatic endocrine dysfunction by potent pancreatic exocrine supplements in patients with chronic pancreatitis. J Clin Gastroenterol 2001; 32: 319-323.
- 55. Stern RC, Eisenberg JD, Wagener JS, et al. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical exocrine pancreatic insufficiency. Am J Gastroenterol 2000; 95: 1932-1938.
- 56. Czakó L, Takács T, Hegyi P, et al. Quality of life assessment after pancreatic enzyme replacement therapy in chronic pancreatitis. Can J Gastroenterol 2003; 17: 597-603.
- 57. Brown A, Hughes M, Tenner S, Banks PA. Does pancreatic enzyme supplementation reduce pain in patients with chronic pancreatitis: a meta-analysis. Am J Gastroenterol 1997; 92: 2032-2035.
- 58. Warshaw AL, Banks, PA, Fernández-Del Castillo C. AGA technical review: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115: 765-776.
- 59. American Gastroenterological Association medical position statement: treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115: 763-764.
- 60. Nydegger A, Couper RT, Oliver MR. Childhood pancreatitis. J Gastroenterol Hepatol 2006; 21: 499-509.
- 61. Campbell D, Jadunandan I, Curington C, et al. Alcoholic and idiopathic patients with painful chronic pancreatitis do not experience suppression of CCK levels or pain relief following treatment with enteric-coated pancreatin [abstract]. Gastroenterology 1992; 102: A259.
- 62. Mössner J, Secknus R, Meyer J, et al. Treatment of pain with pancreatic extracts in chronic pancreatitis: results of a prospective placebo-controlled multicenter trial. Digestion 1992; 53: 54-66.
- 63. Malesci A, Gaia E, Fioretta A, et al. No effect of long-term treatment with pancreatic extract on recurrent abdominal pain in patients with chronic pancreatitis. Scand J Gastroenterol 1995; 30: 392-398.
- 64. Kahl S, Zimmermann S, Leodolter A, et al. Quality of life in patients with chronic pancreatitis after medical treatment. Pancreatology 2001; 1: 149.
- 65. Cystic Fibrosis Australia. About CF. http://www.cysticfibrosis.org.au/aboutcf (accessed Feb 2010).
- 66. Gaskin K, Gurwitz D, Durie P, et al. Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J Pediatr 1982; 100: 857-862.
- 67. Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol 1988; 41: 583-591.
- 68. Lai HC, Corey M, FitzSimmons S, et al. Comparison of growth status of patients with cystic fibrosis between the United States and Canada. Am J Clin Nutr 1999; 69: 531-538.
- 69. Stallings VA, Stark LJ, Robinsons KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc 2008; 108: 832-839.
- 70. Haegel P, Stock C, Marescaux J, et al. Hyperplasia of the exocrine pancreas after small bowel resection in the rat. Gut 1981; 22: 207-212.
- 71. Gelinas MD, Morin CL, Morisset J. Exocrine pancreatic function following proximal small bowel resections in rats. J Physiol 1982; 322: 71-82.
- 72. Friess H, Böhm J, Müller MW, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol 1996; 91: 341-347.
- 73. Friess H, Tempia-Caliera AA, Cammerer G, Büchler MW. Indication for pancreatic enzyme substitution following gastric resection. Pancreatology 2001; 1 Suppl 1: 41-48.
- 74. Gullo L, Costa PL, Ventrucci M, et al. Exocrine pancreatic function after total gastrectomy. Scand J Gastroenterol 1979; 14: 401-407.
- 75. Armbrecht U, Lundell L, Stockbrügger RW. The benefit of pancreatic enzyme substitution after total gastrectomy. Aliment Pharmacol Ther 1988; 2: 493-500.
- 76. Brägelmann R, Armbrecht U, Rosemeyer D, et al. The effect of pancreatic enzyme supplementation in patients with steatorrhoea after total gastrectomy. Eur J Gastroenterol Hepatol 1999; 11: 231-237.
- 77. Friess H, Böhm J, Ebert M, Büchler M. Enzyme treatment after gastrointestinal surgery. Digestion 1993; 54 Suppl 2: 48-53.
- 78. Sategna-Guidetti C, Bianco L. Malnutrition and malabsorption after total gastrectomy. A pathophysiologic approach. J Clin Gastroenterol 1989; 11: 518-524.
- 79. Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol 2004; 18: 947-955.
- 80. Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumours. Br J Surg 2008; 95: 85-91.
- 81. Nakamura Y, Higuchi S, Maruyama K. Pancreatic volume associated with endocrine and exocrine function of the pancreas among Japanese alcoholics. Pancreatology 2005; 5: 422-431.
- 82. Jang JY, Kim SW, Park SJ, Park YH. Comparison of the functional outcome after pylorus-preserving pancreatoduodenectomy: pancreatogastrostomy and pancreatojejunostomy. World J Surg 2002; 26: 366-371.
- 83. Van Hoozen CM, Peeke PG, Taubeneck M, et al. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas 1997; 14: 174-180.
- 84. Neoptolemos JP, Ghaneh P, Andrén-Sandberg A, et al. Treatment of pancreatic exocrine insufficiency after pancreatic resection. Results of a randomized, double-blind, placebo-controlled, crossover study of high vs standard dose pancreatin. Int J Pancreatol 1999; 25: 171-180.
- 85. Smith RC, Talley NJ, Dent OF, et al. Exocrine pancreatic function and chronic unexplained dyspepsia. A case-control study. Int J Pancreatol 1991; 8: 253-262.
- 86. Perez MM, Newcomer AD, Moertel AD, et al. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer 1983; 52: 346-352.
- 87. DiMagno EP, Malagelada JR, Go VL. The relationships between pancreatic ductal obstruction and pancreatic secretion in man. Mayo Clin Proc 1979; 54: 157-162.
- 88. Carroccio A, Iacono G, Montalto G, et al. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut 1991; 32: 796-799.
- 89. Perri F, Pastore M, Festa V, et al. Intraduodenal lipase activity in celiac disease assessed by means of 13C mixed-triglyceride breath test. J Pediatr Gastroenterol Nutr 1998; 27: 407-410.
- 90. Carroccio A, Iacono G, Lerro P, et al. Role of pancreatic impairment in growth recovery during gluten-free diet in childhood celiac disease. Gastroenterology 1997; 112: 1839-1844.
- 91. Carroccio A, Iacono G, Ippolito S, et al. Usefulness of faecal elastase-1 assay in monitoring pancreatic function in childhood coeliac disease. Ital J Gastroenterol Hepatol 1998; 30: 500-504.
- 92. Collins BJ, Bell PM, Boyd S, et al. Endocrine and exocrine pancreatic function in treated coeliac disease. Pancreas 1986; 1: 143-147.
- 93. Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology 1997; 112: 1830-1838.
- 94. Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 2002; 97: 2016-2021.
- 95. Leeds JS, Hopper AD, Hurlstone DP, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther 2007; 25: 265-271.
- 96. Carroccio A, Iacono G, Montalto G, et al. Pancreatic enzyme therapy in childhood celiac disease. A double-blind prospective randomized study. Dig Dis Sci 1995; 40: 2555-2560.
- 97. Czakó L, Hegyi P, Rakonczay Z Jr, et al. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology 2009; 9: 351-359.
- 98. Hardt PD, Krauss A, Bretz L, et al. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol 2000; 37: 105-110.
- 99. Rathmann W, Haastert B, Icks A, et al. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol 2001; 36: 1056-1061.
- 100. Icks A, Haastert B, Giani G, Rathmann W. Low fecal elastase-1 in type 1 diabetes mellitus. Z Gastroenterol 2001; 39: 823-830.
- 101. Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology 2003; 3: 395-402.
- 102. Ewald N, Bretzel RG, Fantus IG, et al. Pancreatin therapy in patients with insulin-treated diabetes mellitus and exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations. Results of a prospective multi-centre trial. Diabetes Metab Res Rev 2007; 23: 386-391.
- 103. Hardt PD, Hauenschild A, Jaeger C, et al. High prevalence of steatorrhea in 101 diabetic patients likely to suffer from exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations: a prospective multicenter study. Dig Dis Sci 2003; 48: 1688-1692.
- 104. Glasbrenner B, Malfertheiner P, Kerner W, et al. [Effect of pancreatin on diabetes mellitus in chronic pancreatitis] [German]. Z Gastroenterol 1990; 28: 275-279.
- 105. Mohan V, Poongothai S, Pitchumoni CS. Oral pancreatic enzyme therapy in the control of diabetes mellitus in tropical calculous pancreatitis. Int J Pancreatol 1998; 24: 19-22.
- 106. Carroccio A, Fontana M, Spagnuolo MI, et al. Pancreatic dysfunction and its association with fat malabsorption in HIV infected children. Gut 1998; 43: 558-563.
- 107. Carroccio A, Di Prima L, Di Grigoli C, et al. Exocrine pancreatic function and fat malabsorption in human immunodeficiency virus-infected patients. Scand J Gastroenterol 1999; 34: 729-734.
- 108. Kapembwa MS, Fleming SC, Griffin GE, et al. Fat absorption and exocrine pancreatic function in human immunodeficiency virus infection. Q J Med 1990; 74: 49-56.
- 109. Price DA, Schmid ML, Ong EL, et al. Pancreatic exocrine insufficiency in HIV-positive patients. HIV Med 2005; 6: 33-36.
- 110. Carroccio A, Guarino A, Zuin G, et al. Efficacy of oral pancreatic enzyme therapy for the treatment of fat malabsorption in HIV-infected patients. Aliment Pharmacol Ther 2001; 15: 1619-1625.
- 111. Leeds JS, Hopper AD, Sidhu R, et al. Some patients with irritable bowel syndrome may have exocrine pancreatic insufficiency. Clin Gastroenterol Hepatol 2010; 8: 433-438.
- 112. Mylvaganam K, Hudson PR, Ross A, Williams CP. 14C triolein breath test: a routine test in the gastroenterology clinic? Gut 1986; 27: 1347-1352.
- 113. Money ME, Hofmann AF, Hagey LR, et al. Treatment of irritable bowel syndrome-diarrhea with pancrealipase or colesevelam and association with steatorrhea. Pancreas 2009; 38: 232-233.





Abstract
Pancreatic exocrine insufficiency (PEI) occurs when the amounts of enzymes secreted into the duodenum in response to a meal are insufficient to maintain normal digestive processes.
The main clinical consequence of PEI is fat maldigestion and malabsorption, resulting in steatorrhoea.
Pancreatic exocrine function is commonly assessed by conducting a 3-day faecal fat test and by measuring levels of faecal elastase-1 and serum trypsinogen.
Pancreatic enzyme replacement therapy is the mainstay of treatment for PEI.
In adults, the initial recommended dose of pancreatic enzymes is 25 000 units of lipase per meal, titrating up to a maximum of 80 000 units of lipase per meal.
In infants and children, the initial recommended dose of pancreatic enzymes is 500 units of lipase per gram of dietary fat; the maximum daily dose should not exceed 10 000 units of lipase per kilogram of bodyweight.
Oral pancreatic enzymes should be taken with meals to ensure adequate mixing with the chyme.
Adjunct therapy with acid-suppressing agents may be useful in patients who continue to experience symptoms of PEI despite high-dose enzyme therapy.
A dietitian experienced in treating PEI should be involved in patient management.
Dietary fat restriction is not recommended for patients with PEI.
Patients with PEI should be encouraged to consume small, frequent meals and to abstain from alcohol.
Medium-chain triglycerides do not provide any clear nutritional advantage over long-chain triglycerides, but can be trialled in patients who fail to gain or to maintain adequate bodyweight in order to increase energy intake.